The Cellular and Molecular Control of Programmed Cell Death
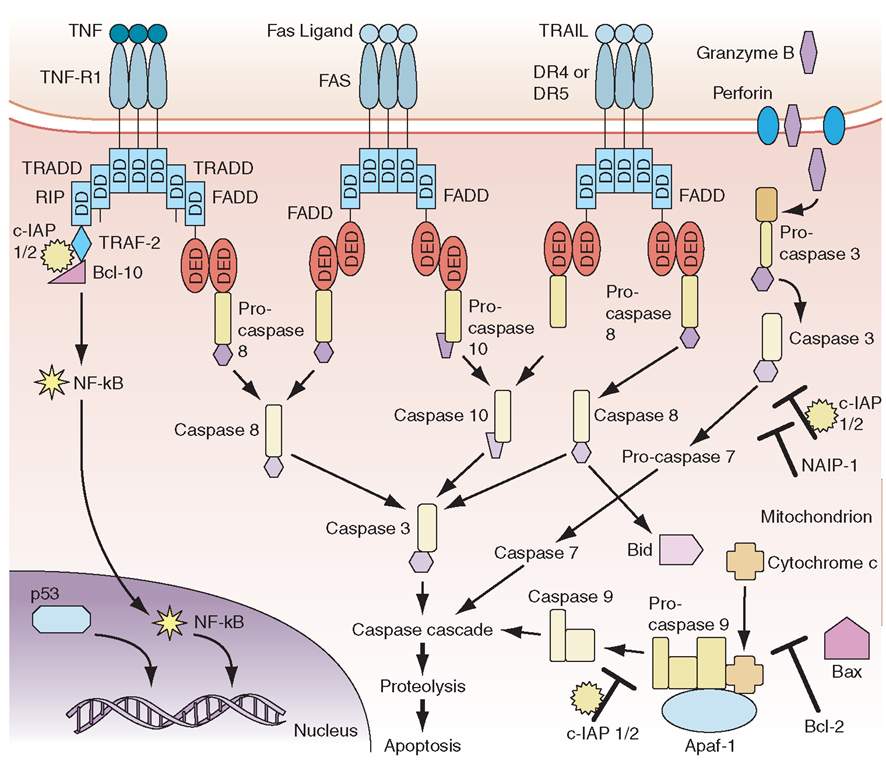
The process of apoptosis (programmed cell death) plays a crucial role in regulating normal immune responses to antigen. In general, a wide variety of stimuli trigger one of several apoptotic pathways to eliminate microbe-infected cells, eliminate cells with damaged DNA, or eliminate activated immune cells that are no longer needed (Fig. 1-10).The largest known family of “death receptors” is the tumor necrosis factor receptor (TNF-R) family [TNF-R1,TNF-R2, Fas (CD95), death receptor 3 (DR3), death receptor 4 (DR4, TRAIL-R1), and death receptor 5 (DR5, TRAIL-R2)]; their ligands are all in the TNF-α family. Binding of ligands to these death receptors leads to a signaling cascade that involves activation of the caspase family of molecules that leads to DNA cleavage and cell death. Two other pathways of programmed cell death involve nuclear p53 in the elimination of cells with abnormal DNA and mitochondrial cytochrome c to induce cell death in damaged cells (Fig. 1-10).A number of human diseases have now been described that result from, or are associated with, mutated apoptosis genes (Table 1-13). These include mutations in the TNF-R1 in hereditary periodic fever (familial Mediterranean fever), Fas and Fas ligand in autoimmune and lymphoproliferation syndromes, and multiple associations of mutations in genes in the apop-totic pathway with malignant syndromes.
Mechanisms of Immune-Mediated Damage to Microbes or Host Tissues
Several responses by the host innate and adaptive immune systems to foreign microbes culminate in rapid and efficient elimination of microbes. In these scenarios, the classic weapons of the adaptive immune system (T cells, B cells) interface with cells (macrophages, dendritic cells, NK cells, neutrophils, eosinophils, basophils) and soluble products (microbial peptides, pentraxins, complement and coagulation systems) of the innate immune system.
FIGURE 1-10
Scheme of major apoptosis pathways. DD, death domain; DED, death effector domain.
There are five general phases of host defenses: (1) migration of leukocytes to sites of antigen localization; (2) antigen-nonspecific recognition of pathogens by macrophages and other cells and systems of the innate immune system; (3) specific recognition of foreign antigens mediated by T and B lymphocytes; (4) amplification of the inflammatory response with recruitment of specific and nonspecific effector cells by complement components, cytokines, kinins, arachidonic acid metabolites, and mast cell-basophil products; and (5) macrophage, neutrophil, and lymphocyte participation in destruction of antigen with ultimate removal of antigen particles by phagocytosis (by macrophages or neutrophils) or by direct cytotoxic mechanisms (involving macrophages, neutrophils, DCs, and lymphocytes). Under normal circumstances, orderly progression of host defenses through these phases results in a well-controlled immune and inflammatory response that protects the host from the offending antigen. However, dysfunction of any of the host defense systems can damage host tissue and produce clinical disease. Furthermore, for certain pathogens or antigens, the normal immune response itself might contribute substantially to the tissue damage. For example, the immune and inflammatory response in the brain to certain pathogens such as M. tuberculosis may be responsible for much of the morbidity of this disease in that organ system. In addition, the morbidity associated with certain pneumonias such as that caused by Pneumocystis carinii may be associated more with inflammatory infiltrates than with the tissue-destructive effects of the microorganism itself.
The Molecular Basis of Lymphocyte-Endothelial Cell Interactions
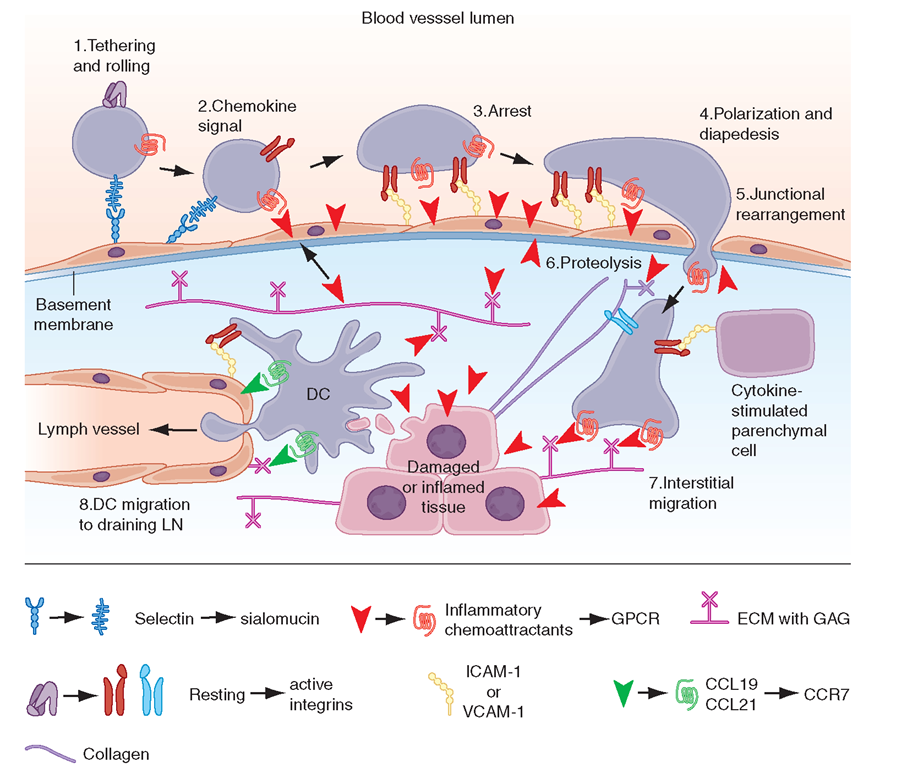
The control of lymphocyte circulatory patterns between the bloodstream and peripheral lymphoid organs operates at the level of lymphocyte-endothelial cell interactions to control the specificity of lymphocyte subset entry into organs. Similarly, lymphocyte-endothelial cell interactions regulate the entry of lymphocytes into inflamed tissue. Adhesion molecule expression on lymphocytes and endothelial cells regulates the retention and subsequent egress of lymphocytes within tissue sites of antigenic stimulation, delaying cell exit from tissue and preventing reentry into the circulating lymphocyte pool (Fig. 1-11). All types of lymphocyte migration begin with lymphocyte attachment to specialized regions of vessels, termed high endothelial venules (HEVs).An important concept is that adhesion molecules do not generally bind their ligand until a conformational change (ligand activation) occurs in the adhesion molecule that allows ligand binding. Induction of a conformation-dependent determinant on an adhesion molecule can be accomplished by cytokines or via ligation of other adhesion molecules on the cell.
FIGURE 1-11
Key migration steps of immune cells at sites of inflammation. Inflammation due to tissue damage or infection induces the release of cytokines (not shown) and inflammatory chemoattractants (red arrowheads) from distressed stromal cells and “professional” sentinels, such as mast cells and macrophages (not shown). The inflammatory signals induce upregulation of endothelial selectins and immunoglobulin “superfamily” members, particularly ICAM-1 and/or VCAM-1. Chemoattractants, particularly chemokines, are produced by or translocated across venular endothelial cells (red arrow) and are displayed in the lumen to rolling leukocytes. Those leukocytes that express the appropriate set of trafficking molecules undergo a multistep adhesion cascade (steps 1-3) and then polarize and move by dia-pedesis across the venular wall (steps 4 and 5). Diapedesis involves transient disassembly of endothelial junctions and penetration through the underlying basement membrane (step 6). Once in the extravascular (interstitial) space, the migrating cell uses different integrins to gain “footholds” on collagen fibers and other ECM molecules, such as laminin and fibronectin, and on inflammation-induced ICAM-1 on the surface of parenchymal cells (step 7). The migrating cell receives guidance cues from distinct sets of chemoattrac-tants, particularly chemokines, which may be immobilized on glycosaminoglycans (GAG) that “decorate” many ECM molecules and stromal cells. Inflammatory signals also induce tissue-resident DCs to undergo maturation. Once DCs process material from damaged tissues and invading pathogens, they upregulate CCR7, which allows them to enter draining lymph vessels that express the CCR7 ligancd CCL21 (and CCL19). In lymph nodes (LN), these antigen-loaded mature DCs activate naïve T cells and expand pools of effector lymphocytes, which enter the blood and migrate back to the site of inflammation. T cells in tissue also use this CCR7-dependent route to migrate from peripheral sites to draining lymph nodes through afferent lymphatics.
The first stage of lymphocyte-endothelial cell interactions, attachment and rolling, occurs when lymphocytes leave the stream of flowing blood cells in a postcapillary venule and roll along venule endothelial cells (Fig. 1-11). Lymphocyte rolling is mediated by the L-selectin molecule (LECAM-1, LAM-1, CD62L) and slows cell transit time through venules, allowing time for activation of adherent cells.
The second stage of lymphocyte-endothelial cell interactions, firm adhesion with activation-dependent stable arrest,requires stimulation of lymphocytes by chemoattrac-tants or by endothelial cell-derived cytokines. Cytokines thought to participate in adherent cell activation include members of the IL-8 family, platelet-activation factor, leukotriene B4, and C5a. In addition, HEVs express chemokines, SLC (CCL21) and ELC (CCL19), which participate in this process. Following activation by chemoattractants, lymphocytes shed L-selectin from the cell surface and upregulate cell CD11b/18 (MAC-1) or CD11a/18 (LFA-1) molecules, resulting in firm attachment of lymphocytes to HEVs.
Lymphocyte homing to peripheral lymph nodes involves adhesion of L-selectin to glycoprotein HEV ligands collectively referred to as peripheral node addressin (PNAd), whereas homing of lymphocytes to intestine Peyer’s patches primarily involves adhesion of the a4,ß7 integrin to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on the Peyer’s patch HEVs. However, for migration to mucosal Peyer’s patch lymphoid aggregates, naïve lymphocytes primarily use L-selectin, whereas memory lymphocytes use a4,ß7 integrin. a4,ß1 inte-grin (CD49d/CD29,VLA-4)-VCAM-1 interactions are important in the initial interaction of memory lymphocytes with HEVs of multiple organs in sites of inflammation (Table 1-14).
The third stage of leukocyte emigration in HEVs is sticking and arrest. Sticking of the lymphocyte to endothelial cells and arrest at the site of sticking are mediated predominantly by ligation of aL,ß2 integrin LFA-1 to the integrin ligand ICAM-1 on HEVs. While the first three stages of lymphocyte attachment to HEVs take only a few seconds, the fourth stage of lymphocyte emigration, transendothelial migration, takes —10 min. Although the molecular mechanisms that control lymphocyte transendothelial migration are not fully characterized, the HEV CD44 molecule and molecules of the HEV glyco-calyx (extracellular matrix) are thought to play important regulatory roles in this process (Fig. 1-11). Finally, expression of matrix metalloproteases capable of digesting the subendothelial basement membrane, rich in nonfibrillar collagen, appears to be required for the penetration of lymphoid cells into the extravascular sites.
Abnormal induction of HEV formation and use of the molecules discussed above have been implicated in the induction and maintenance of inflammation in a number of chronic inflammatory diseases. In animal models of Type 1 diabetes mellitus, MAdCAM-1 and GlyCAM-1 have been shown to be highly expressed on HEVs in inflamed pancreatic islets, and treatment of these animals with inhibitors of L-selectin and a4 integrin function blocked the development of Type 1 diabetes mellitus. A similar role for abnormal induction of the adhesion molecules of lymphocyte emigration has been suggested in rheumatoid arthritis (Chap. 5), Hashimoto’s thyroiditis, Graves’ disease, multiple sclerosis, Crohn’s disease, and ulcerative colitis.
Immune-Complex Formation
Clearance of antigen by immune-complex formation between antigen and antibody is a highly effective mechanism of host defense. However, depending on the level of immune complexes formed and their physicochemical properties, immune complexes may or may not result in host and foreign cell damage. After antigen exposure, certain types of soluble antigen-antibody complexes freely circulate and, if not cleared by the reticuloendothelial system, can be deposited in blood vessel walls and in other tissues such as renal glomeruli and cause vasculitis or glomerulonephritis syndromes (Chap. 10).
Immediate-Type Hypersensitivity
Helper T cells that drive antiallergen IgE responses are usually TH2-type inducer T cells that secrete IL-4, IL-5, IL-6, and IL-10. Mast cells and basophils have high-affinity receptors for the Fc portion of IgE (FcRI), and cell-bound antiallergen IgE effectively “arms” basophils and mast cells. Mediator release is triggered by antigen (allergen) interaction with Fc receptor-bound IgE; the mediators released are responsible for the pathophysiologic changes of allergic diseases (Table 1-10). Mediators released from mast cells and basophils can be divided into three broad functional types: (1) those that increase vascular permeability and contract smooth muscle (histamine, platelet-activating factor, SRS-A, BK-A), (2) those that are chemotactic for or activate other inflammatory cells (ECF-A, NCF, leukotriene B4), and (3) those that modulate the release of other mediators (BK-A, platelet-activating factor).
Cytotoxic Reactions of Antibody
In this type of immunologic injury, complement-fixing (C1-binding) antibodies against normal or foreign cells or tissues (IgM, IgG1, IgG2, IgG3) bind complement via the classic pathway and initiate a sequence of events similar to that initiated by immune-complex deposition, resulting in cell lysis or tissue injury. Examples of antibody-mediated cytotoxic reactions include red cell lysis in transfusion reactions, anti-glomerular basement disease (Goodpasture’s syndrome) with anti-glomerular basement membrane antibody formation, and pemphigus vulgaris with antiepidermal antibodies inducing blistering skin disease.
Classic Delayed-Type Hypersensitivity Reactions
Inflammatory reactions initiated by mononuclear leukocytes and not by antibody alone have been termed delayed-type hypersensitivity reactions. The term delayed has been used to contrast a secondary cellular response that appears 48-72 h after antigen exposure with an immediate hypersensitivity response generally seen within 12 h of antigen challenge and initiated by basophil mediator release or preformed antibody. For example, in an individual previously infected with M. tuberculosis organisms, intradermal placement of tuberculin purified-protein derivative as a skin test challenge results in an indurated area of skin at 48-72 h, indicating previous exposure to tuberculosis.
TABLE 1-14
|
TRAFFICKING MOLECULES INVOLVED IN INFLAMMATORY DISEASE PROCESSES |
||||
|
|
PROPOSED LEUKOCYTE RECEPTORS FOR ENDOTHELIAL TRAFFIC SIGNALS |
|||
|
DISEASE |
KEY EFFECTOR CELL |
L-SELECTIN, LIGAND |
GPCR |
INTEGRINa |
|
Acute Inflammation |
||||
|
Myocardial infarction |
Neutrophil |
PSGL-1 |
CXCR1, CXCR2, PAFR, BLT1 |
LFA-1, Mac-1 |
|
Stroke |
Neutrophil |
L-selectin, PSGL-1 |
CXCR1, CXCR2, PAFR, BLT1 |
LFA-1, Mac-1 |
|
Ischemia-reperfusion |
Neutrophil |
PSGL-1 |
CXCR1, CXCR2, PAFR, BLT1 |
LFA-1, Mac-1 |
|
TH1 Inflammation |
||||
|
Atherosclerosis |
Monocyte |
PSGL-1 |
CCR1, CCR2, BLT1, CXCR2, CX3CR1 |
VLA-4 |
|
TH1 |
PSGL-1 |
CXCR3, CCR5 |
VLA-4 |
|
|
Multiple sclerosis |
TH1 |
PSGL-1 (?) |
CXCR3, CXCR6 |
VLA-4, LFA-1 |
|
Monocyte |
PSGL-1 (?) |
CCR2, CCR1 |
VLA-4, LFA-1 |
|
|
Rheumatoid arthritis |
Monocyte |
PSGL-1 |
CCR1, CCR2 |
VLA-1, VLA-2, VLA-4, |
|
LFA-1 |
||||
|
TH1 |
PSGL-1 |
CXCR3, CXCR6 |
VLA-1, VLA-2, VLA-4, |
|
|
LFA-1 |
||||
|
Neutrophil |
L-selectin, PSGL-1 |
CXCR2, BLT1 |
LFA-1 h |
|
|
Psoriasis |
Skin-homing TH1 |
CLA |
CCR4, CCR10, CXCR3 |
VLA-4,c LFA-1 |
|
Crohn’s disease |
Gut-homing TH1 |
PSGL-1 |
CCR9, CXCR3 |
α4β7, LFA-1 |
|
Type I diabetes |
TH1 |
PSGL-1 (?) |
CCR4, CCR5 |
VLA-4, LFA-1 |
|
CD8 |
L-selectin (?), |
CXCR3 |
VLA-4, LFA-1 |
|
|
PSGL-1 (?) |
||||
|
Allograft rejection |
CD8 |
PSGL-1 |
CXCR3, CX3CR1, BLT1 |
VLA-4, LFA-1 |
|
B cell |
L-selectin, PSGL-1 |
CXCR5, CXCR4 |
VLA-4, LFA-1 |
|
|
Hepatitis |
CD8 |
PSGL-1 |
CXCR3, CCR5, CXCR6 |
VLA-4 |
|
Lupus |
TH1 |
None |
CXCR6 |
VLA-4d |
|
Plasmacytoid DC |
L-selectin, CLA |
CCR7, CXCR3, ChemR23 |
LFA-1, Mac-1 |
|
|
B cell |
CLA (?) |
CXCR5, CXCR4 |
LFA-1 |
|
|
TH2 Inflammation |
||||
|
Asthma |
TH2 |
PSGL-1 |
CCR4, CCR8, BLT1 |
LFA-1 |
|
Eosinophil |
PSGL-1 |
CCR3, PAFR, BLT1 |
VLA-4, LFA-1 |
|
|
Mast cells |
PSGL-1 |
CCR2, CCR3, BLT1 |
VLA-4, LFA-1 |
|
|
Atopic dermatitis |
Skin-homing TH2 |
CLA |
CCR4, CCR10 |
VLA-4, LFA-1 |
aVarious ß1 integrins have been linked in different ways in basal lamina and interstitial migration of distinct cell types and inflammatory settings.
hIn some settings, Mac-1 has been linked to transmigration.
cCD44 can act in concert with VLA-4 in particular models of leukocyte arrest.
dTH2 cells require VAP-1 to traffic to inflamed liver.
The cellular events that result in classic delayed-type hypersensitivity responses are centered around T cells (predominantly, though not exclusively, IFN-γ, IL-2.Recently NK cells have been suggested to play a major role in the form of delayed hypersensitivity that occurs following skin contact with immunogens. First, local immune and inflammatory responses at the site of foreign antigen upregulate endothelial cell adhesion molecule expression, promoting the accumulation of lymphocytes at the tissue site. In the general schemes outlined in Figs. 1-2 and 1-3, antigen is processed by dendritic cells and presented to small numbers of CD4+ T cells expressing a TCR specific for the antigen. IL-12 produced by APCs induces T cells to produce IFN-γ (TH1 response). Macrophages frequently undergo epithelioid cell transformation and fuse to form multinucleated giant cells in response to IFN-γ. This type of mononuclear cell infiltrate is termed granulomatous inflammation. Examples of diseases in which delayed-type hypersensitivity plays a major role are fungal infections (histoplasmosis), mycobacterial infections (tuberculosis, leprosy), chlamydial infections (lymphogranuloma venereum), helminth infections (schistosomiasis), reactions to toxins (berylliosis), and hypersensitivity reactions to organic dusts (hypersensitivity pneumonitis). In addition, delayed-type hypersensitivity responses play important roles in tissue damage in autoimmune diseases such as rheumatoid arthritis, giant cell arteritis, and Wegener’s granulomatosis.