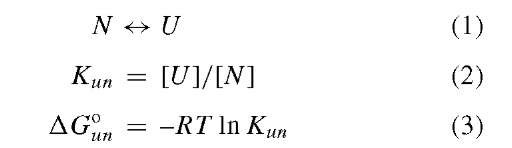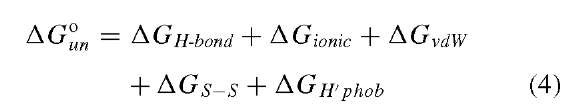Stability of a protein is usually studied by observing the energetics of unfolding transitions given by the equations below:
These equations apply to a simple two-state transition between the native (N) and the unfolded (U) state given by the equilibrium constant Kun. This is, by definition, a cooperative process without a detectable intermediate species. The denatured or unfolded state of a protein is generally considered to be an ensemble of conformations in which all parts of the protein are exposed to the solvent with a minimum of intramolecular interactions. The denatured state has high conformational entropy and is biologically inactive. The unfolding transition (Eq. (1) and Fig. 2) can be induced by pressure, temperature, extreme pH, and denaturants such as urea and guanidine HCl, as will discussed in a subsequent section. These perturbants disrupt the intramolecular interactions that hold proteins together. One can imagine that the ensemble of unfolded states could be influenced by the means used to unfold.
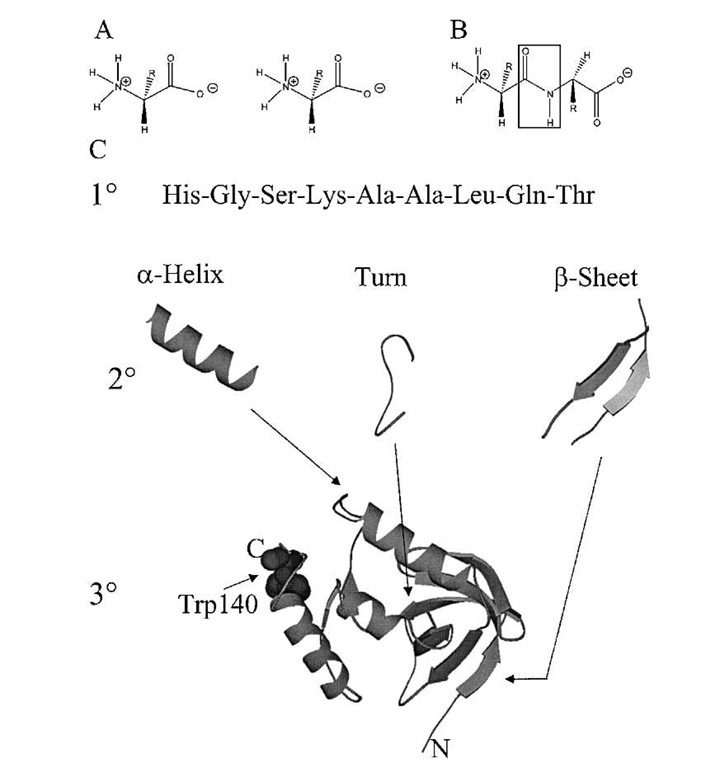
FIGURE 1 Diagram of the levels of protein structure. (A) Amino acids are the basic building blocks (monomers) of proteins (polymers). All amino acids contain a carboxylic acid group and an amine group connected by a central carbon called the a-carbon. Each of the 20 common amino acids, designated by a three-letter code, has a unique side chain (R) that is also bonded to the a-carbon. (B) Through a dehydrolysis reaction, an amide bond is formed (boxed region) that links the amino group of one amino acid to the carboxylic acid group of the next amino acid. (C) The primary structure of proteins (1°) is the linear sequence of amino acids written from the amino-terminal end (left) to the carboxy-terminal end (right), by convention. Secondary structure of proteins (2°) is classified into three major categories. In the a-helix structure, the backbone atoms (amide linkage and a-carbon) coil into a right-handed helical shape (residues 55 to 67 from staphylococcal nuclease1 are shown). The a-helix is held together through a series of hydrogen bonds between the amide hydrogen and the carboxyl oxygen of the backbone atoms from amino acids further up the chain. The side chains (not shown) protrude from the central core structure like the spokes of a wheel. Another important secondary structure type is the turn (residues 76 to 88 from Staphyloccocal nuclease are shown). We use the term turn loosely here to represent the regions of proteins that turn corners, thereby allowing interactions between different and often distant (in terms of primary structure) substructures. The p-sheet structure is the third common secondary structure type (residues 9 to 12 and 72 to 76 from Staphylococcal nuclease are shown). It is similar to the a-helical structure in that hydrogen bonds between backbone atoms hold the structure together and the side chains (not shown) protrude from the structure above and below the plane of the sheet. In contrast to the a-helix, the p-sheet can be formed from segments of protein that are far apart in the primary sequence. The tertiary structure (3°) is the three-dimensional association of secondary structures into a unique and stable final fold. A ribbon tracing the backbone atoms of Staphylococcal nuclease is shown.1-2. The N-terminus of the protein is in the bottom right and the C-terminus is in the top left of the figure. No side chains are drawn except that of residues tryptophan 140.
The native structure of proteins is stabilized by intramolecular, noncovalent interactions including hydrogen bonding, ionic, and van der Waals interactions, and covalent cross-links (disulfide bridges between cysteine residues) according to Eq. (4):
Each term in Eq. (4) will be discussed separately. As mentioned earlier, an important stabilizing factor for the tertiary fold of a protein is its intramolecular hydrogen bonds (AGH-bond). Secondary structures are stabilized by hydrogen bonds between backbone amide atoms (Fig. 1). The side chains of neighboring secondary structural units can interact through hydrogen bonding. Ionic interactions (AGionic) between acidic and basic side chains may stabilize the tertiary structure of proteins and are pH dependent. The actual pKa of an ionizable side chain is influenced by the microenvironment in which it resides. Nonpolar and polar, but uncharged, amino acids interact through van der Waals interactions (AGvdW). In some proteins, cysteine residues (side chain is a sulfhydryl) form disulfide linkages that can increase the overall stability of the protein (AGS-S). Other possible factors not considered explicitly here are the effects of metals, nucleotides, prosthetic groups, and cofactors on protein structure and stability.
By far the most important noncovalent factor that determines protein stability is hydrophobic interactions (AGHphob). In globular proteins, hydrophobic amino acids are buried in the interior where they create a "hydrophobic core." Although these nonpolar residues participate in van der Waals interactions, the primary driving force for the formation of the hydrophobic core is to avoid the aqueous solvent. Solvation of nonpolar side chains by aqueous solutions causes a decrease in the entropy of solution. To avoid this entropic penalty, proteins typically bury their nonpolar residues in the interior of a protein.4
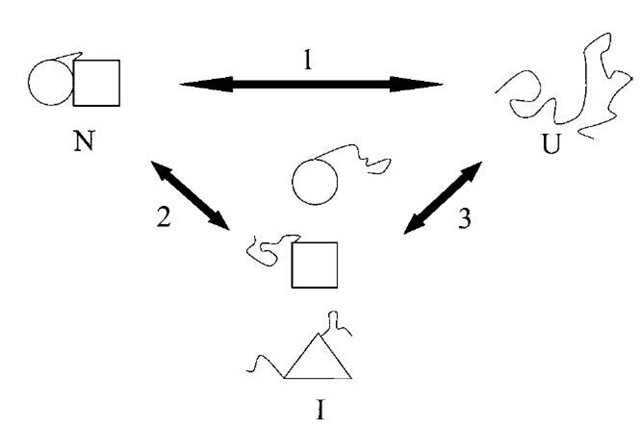
FIGURE 2 Illustration of cooperative vs. noncooperative unfolding transitions. If the native state of a protein (N) is denatured into the unfolded state (U) in a single transition (pathway 1), then it is a two-state or cooperative unfolding transition. Alternatively, the native state may be converted into one or more intermediate states (pathway 2). For example, if a protein is comprised of multiple domains, one of the domains may be unfolded first. It is also possible to form a completely different intermediate before unfolding completely. The presence of intermediate species may be observed using kinetic or equilibrium techniques. However, intermediates detectable by kinetic methods may or may not be observable by equilibrium methods.