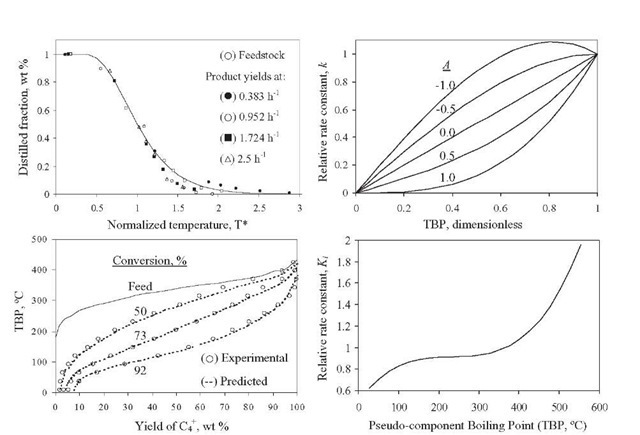
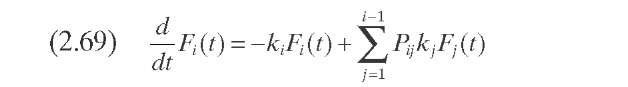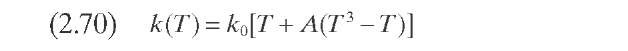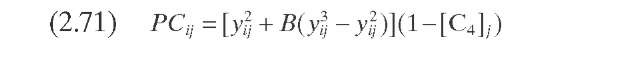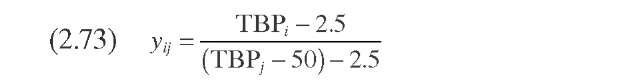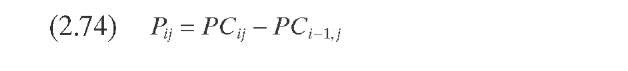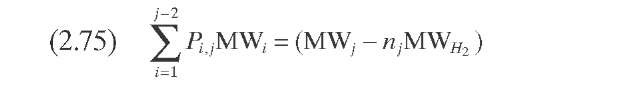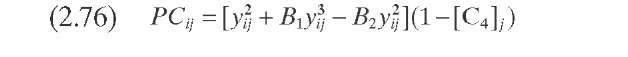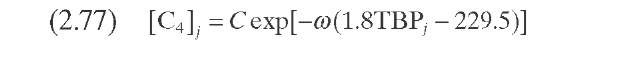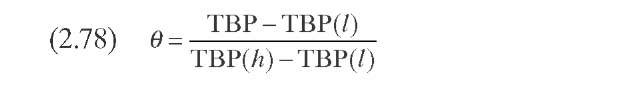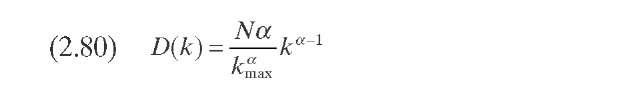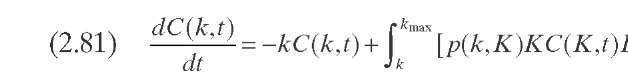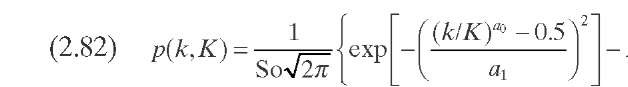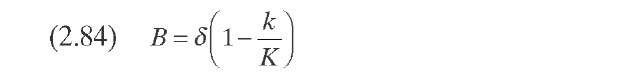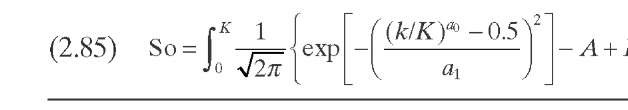Stangeland (1974) developed a kinetic model for predicting hydrocracker yields using correlations based on the boiling point of each of the pseudocom-ponents that characterize the cut. The model includes four parameters: k0 and A quantify each pseudocomponent ‘s reaction rate, C gives the butane yield magnitude, and B varies with both the type of feed (naphtenic or paraffinic) and the type of catalytic process (random or selective). Parameters B and C determine the shape of the yield curve. Although A usually lies in the range 0 to 1.0, it can take negative values. Parameter A determines the shape of the reactivity curve, which varies from a linear to a cubic function, as shown in Figure 2.11- The complete set of equations, presented in Table 2.10 – permits calculation of the formation of component i due to the decomposition of heavy components j.
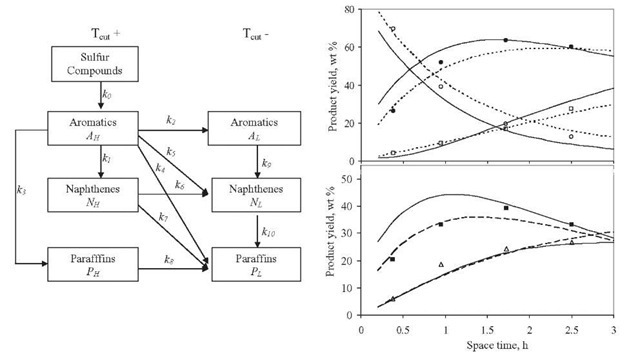
Figure 2.10. Reaction scheme for hydrocracking proposed by Krishna and Saxena ![]() (1989), and comparison of calculated and experimental yields
(1989), and comparison of calculated and experimental yields
![]() dispersion model; – - – , kinetic model).
dispersion model; – - – , kinetic model).
Figure 2.11. Normalized TBP curves, cracking rate function, and comparison of yields for once-through hydrocracking (Stangeland, 1974) and the relative rate constant function (Mohanty et al., 1991).
Sets of data at three conversion levels are illustrated in Figure 2.11 for hydrocracking of raw California gas oil in once-through liquid operation. The yields predicted based on these parameters are shown as dashed lines for conversions of 50, 73, and 92% (288°C-). In general, the agreement with experimental data is quite good and the differences are probably within experimental error. The major disadvantage of this approach is that a change in hydrocracker product specifications, or in the number of products, requires reformulating the model and refitting the data.
Mohanty et al. (1991) implemented Stangeland -s kinetic model in a computer model for a two-stage commercial-scale VGO hydrocracker. The feed and products were lumped into 23 pseudocomponents for the hydrocracking reactions, and pseudohomogeneous first-order reactions were assumed. Estimation of the hydrocracking kinetic constants for the other pseudocom-ponents that comprise the VGO was done with Eq. (2.66), where K was adjusted with plant data using Eq. (2.67) (Figure 2.11). A hydrocracking kinetic constant of vacuum gas oil with an average boiling point of 365°C reported by Qader and Hill (1969) was employed [Eq. (2.68)]. Calculated yields, hydrogen consumption, and outlet temperatures with this model are tabulated in Table 2.9. The model was validated against plant data and the agreement was generally good. It is important to indicate that with the parameters reported by Mohanty et al. (1991), the mass balance closure in each individual hydro-cracking reaction is not satisfied.
Dassori and Pacheco (2002) established a link between the stoichiometric coefficient of the hydrocracking reactions and the parameters P tj of Stangeland’s kinetic model; this analogy imposes a constraint on the values that the Pv matrix can take. Such a constraint is given by the mass balance closure in each hydrocracking reaction and would require determination of the values of parameters B and C (Table 2.10). It was noticed that only with these two parameters is it not possible to rearrange the product distribution to satisfy the mass balance for each reaction. These authors modified the model proposed by Stangeland by adding two additional parameters, B2 and a>- so that the mass balance in each hydrocracking reaction is satisfied. They used a second-order hydrocracking rate constant to quantify the effect of hydrogen partial pressure on the rate of cracking. The kinetic constants are determined from the pseudo-first-order constants reported by Qader and Hill (1969). This model was applied to the hydrocracking of VGO in a commercial reactor described by Mohanty et al. (1991).
TABLE 2.10. Equations of the Kinetic Models for Hydrocracking Based on TBP of Pseudocomponents and on Continuous Mixtures
Models Based on Continuous Mixtures
Laxminarasimhan and Verma (1996) developed a kinetic model for hydro-cracking of a petroleum mixture based on the continuous theory of lumping. The model considers properties of the reaction mixture, the underlying pathways, and the associated selectivity of the reactions. The parameter of characterization is the TBP temperature. During the reaction of a particular feed, the mixture’s distillation curve changes continuously inside the reactor, and as the residence time increases, most of the heavier components are converted into lighter components. A normalized TBP as a function of an index (0) is used instead of the TBP. Normalized TBP is defined by Eq. (2.78) (Table 2.10). The reactivity is considered to be monotonic and can be represented by a simple power-law type of function [Eq. (2.79)], where k is the reaction rate of a particular compound, kmax is the reaction rate of the compound of higher TBP, and a is a model parameter. The model equations are formulated as a function of reactivity following a procedure proposed by Chou and Ho (1989). To express the equation with k as the independent reactivity, a transformation operator is required, which is approximated by Eq. (2.80)- D(k) can be considered as a species-type distribution function, where N is the number of compounds in the mixture and tends toward infinitum in a heavy fraction of oil. A material balance of species of reactivity k, the core of the kinetic model, can be expressed with integrodifferential equation (2.81).
P(k,K) is ideally the yield distribution function that describes the formation of compounds of reactivity k from hydrocracking of compounds of reactivity K- This function is approximated in this model by a skewed Gaussian-type distribution function obtained from experimental data on the reactivity of several model compounds [Eqs. (2.82) to (2.85)]. The parameters a0, a1, and 8 are specific for each system and are used for model tuning.
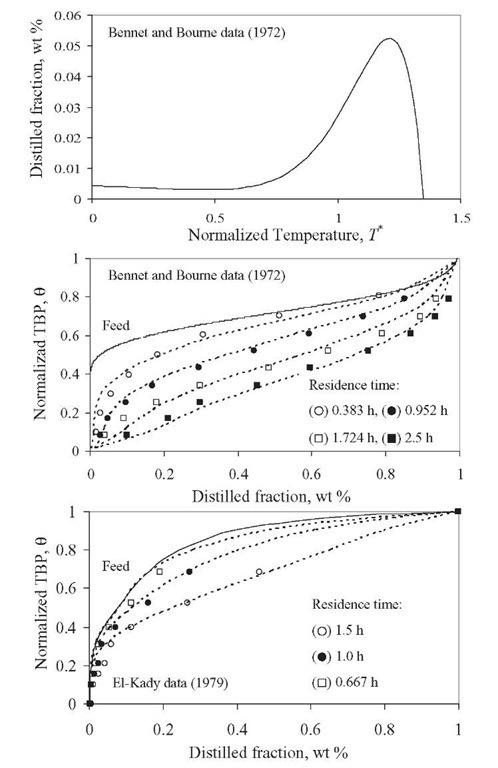
This model was employed successfully to experimental data published previously. Bennett and Bourne (1972) reported product yields from the hydro-cracking of Kuwait vacuum gas oil at four different residence times: 0.383, 0.952, 1.724, and 2.5 h. The set of parameters was tuned using data obtained at 2.5 h of residence time. These parameters are a= 1.35, kmax = 1.35h-1,a0 = 6.41, a1 = 28.15, and 8= 2.6667 x 10-5- Figure 2.12 shows a typical p(k,K) function, in this case, k = kmax. Comparisons of experimental and estimated values are also shown in Figure 2.12 .
El-Kady (1979) reported another set of experimental data at several reaction temperatures and residence times for the hydrocracking of a vacuum gas oil. In Figure 2.12 – comparisons of these experimental data and estimated values at 390°C are presented. The set of fitted parameters of the model reported by Laxminarasimhan and Verma (1996) is a= 0.77, kmax = 0.88 h-1, a0 = 3.67, a1 = 22.86, and 8= 0.77 x 10-9 for experimental data at 390°C.
Extensions of the Laxminarasimhan and Verma (1996) model, in which the reacting mixture is divided into continuous mixtures of paraffinic, naphthenic, and aromatics components, have been published by the same research group (Narasimhan et al., 1997- Basak et al., 2004). In addition to the reactions of hydrocracking that form compounds in the same family, the formation reactions of paraffins from naphthenes, paraffins from aromatics, and naphthenes from aromatics were considered. Therefore, the models require the definition of a concentration function, a reactivity function, and a species distribution function for each family of compounds, as well as six different product distribution functions. The models were validated with the pilot-plant experimental data reported by Bennett and Bourne (1972) – However, parameters of the model functions were not reported. Elizalde et al. (2009) recently reported application of the continuous lumping approach for modeling of hydrocrack-ing of heavy crude oil at moderate reaction conditions. The model parameters were estimated from experiments obtained in an isothermal fixed-bed reactor at 380 to 420°C, 0.33 to 1.5 h-1 LHSV at constant pressure (9.8MPa), and an H2/oil ratio of 5000 ft3/bbl. More details were provided about values and estimation of model parameters. Comparisons between experimental data and predictions using the continuous lumping kinetic model were reported to show good agreement with an average absolute error of less than 5%.
Figure 2.12. Yield distribution function p(k,K) and comparison of estimations (lines) with Laxminarasimhan and Verma model (1996) and experimental data (symbols).
Structure-Oriented Lumping and Single-Event Models
Structure-oriented lumping kinetic models, which employ most of the information obtained through modern analytical techniques for model reaction modeling at a molecular level, have been proposed for some catalytic processes. The lumps are defined according to the structure of the compounds in the reacting mixture.
Liguras and Allen (1989) utilized contribution group concepts, which provide a mechanism for making use of pure compound data in modeling complex reactions. They describe the conversion of vacuum gas oil in terms of a relatively large number of pseudocomponents, most of which are lumps in their own right. Quann and Jaffe (1992) developed a procedure to describe molecules and reactions with a notation of vectors which allows a computer program to represent the reaction networks. These authors expressed the chemical transformations in terms of a typical structure of the molecules without completely eliminating lumps and rate parameters, which depend on the feedstock composition.
Martens and Marin (2001) reported a model for the hydrocracking of hydrogenated vacuum gas oil based on theoretical and mechanistic considerations. The reaction mechanism is described by a set of single events, each of which can be ascribed a rate equation or a term in a single rate equation. The model considers the reaction rules for carbenium ion of the secondary and tertiary types. A computer algorithm was used for generating the reaction networks.
Froment (2005) has recently reviewed the single- event approach, which retains the full details of the reaction pathways of the individual feed components and reaction intermediates. This approach is illustrated by means of the methanol-to-olefins and catalytic cracking of oil fraction reactions. It is also highlighted the fact that other important processes with complex feedstocks, such as catalytic reforming, hydrocracking, alkylation, and isomerization, can be modeled by means of the single-event concept. As can be seen, all these approaches have been used successfully for some complex reaction systems. However, hydrocracking kinetics of heavy oil fractions with structure-oriented lumping modeling or the single-event approaches has not been reported in the open literature.
Lump models have been used for several years for kinetic modeling of complex reactions. In fact, some commercial catalytic process design is still being performed with this type of approach. Catalyst screening, process control, basic process studies, and dynamic modeling, among others, are areas in which lump kinetic models are employed extensively. The main disadvantages of lump models are their simplicity in predicting product yields, the dependency of kinetic parameters on feed properties, and the use of an invariant distillation range of products, which, if changed, necessitates further experiments and parameter estimation.
Models based on continuous mixtures (continuous theory of lumping) overcome some of these deficiencies by considering the properties of the reaction mixture, the underlying pathways, and the associated selectivity of the reactions. The common parameter of characterization is the true boiling- point temperature, since during reaction it changes continuously inside the reactor as the residence time increases. However, the dependency of model parameters on feed properties is still present. Distillation curves, either chromato-graphic or physical, also present some difficulties when analyzing heavy oils, since initial and final boiling points are not accurate during experimentation. In fact, for many purposes, 10% and 90% boiling points are commonly utilized instead of IBP and FBP, respectively.
Structure-oriented lumping models are more detailed approaches that express the chemical transformations in terms of typical molecular structures. These models describe reaction kinetics in terms of a relatively large number of pseudocomponents, and hence they do not completely eliminate lumps. In addition, dependency of rate parameters on feed properties is present.
The single-event concept,which uses elementary steps of cation chemistry, consists of a limited number of types of steps involving a series of homologous species. The number of rate coefficients to be determined from experimental information can be reduced and are modeled based on transition-state theory and statistical thermodynamics. With this approach, parameter values are not dependent on feed properties. However, even though the number of parameters can be diminished, detailed and sufficient experimental data are necessary.
The complexity of real feedstocks suggests that models based on lumping theory will continue to be used for the study of hydrocracking reaction kinetics. However, more sophisticated and accurate approaches need to be studied with more detail for a better understanding and representation of heavy oil hydrocracking kinetics.