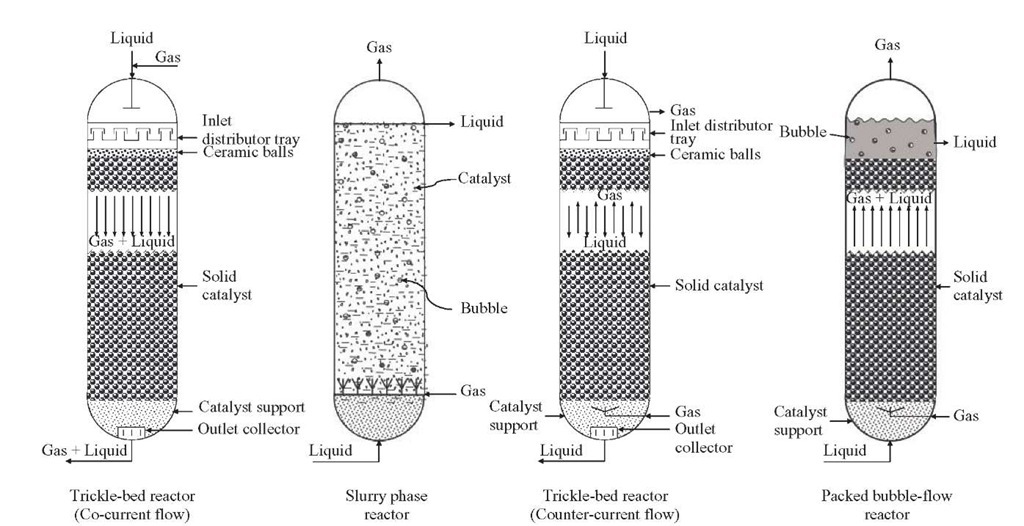
Multiphase catalytic packed-bed reactors (PBRs) operate in two modes: (1) trickle operation, with a continuous gas phase and a distributed liquid phase, and the main mass transfer resistance located in the gas, and (2) bubble operation, with a distributed gas and a continuous liquid phase, and the main mass transfer resistance located in the liquid phase. For three-phase reactions (gas and liquid phases in contact with a solid catalyst), the common modes of operation are trickle- or packed-bed reactors, in which the catalyst is stationary, and slurry reactors, in which the catalyst is suspended in the liquid phase (Figure 2.1). In these reactors, gas and liquid move co-currently down flow or gas is fed countercurrently upflow. Commercially, the former is the most used reactor, in which the liquid phase flows mainly through the catalyst particles in the form of films, rivulets, and droplets (Figure 2.2).
Based on the direction of the fluid flow, PBRs can then be classified as trickle-bed reactors (TBRs) with co-current gas-liquid downflow, trickle-bed reactors with countercurrent gas-liquid flow, and packed-bubble reactors, where gas and liquid are contacted in co-current upflow. To carry out the catalyst and reactor selection and process design properly, knowledge of what each reactor type can and cannot do is very important. When a fixed-bed reactor is chosen, the question frequently asked is whether to use an upflow or downflow mode of operation.
Figure 2.1. Various types of multiphase catalytic reactors.
Figure 2.2. Liquid flow texture found during the trickle-flow regime in a TBR.
In the case of catalytic packed beds with two-phase flow, such as those used for straight-run naphtha hydrodesulfurization, from a reaction engineering perspective, a large catalyst-to-liquid volume ratio and plug flow of both phases are preferred, and catalyst deactivation is very slow or negligible, which facilitates reactor modeling and design. However, for three -phase catalytic reactors such as those employed for hydrotreating of middle distillates and heavy petroleum fractions, the reaction occurs between the dissolved gas and the liquid-phase reactant at the surface of the catalyst, and the choice of upflow versus downflow operation can be based on rational considerations regarding the limiting reactant at the operating conditions of interest (Dudukovic et al., 2002).
Fixed-Bed Reactors
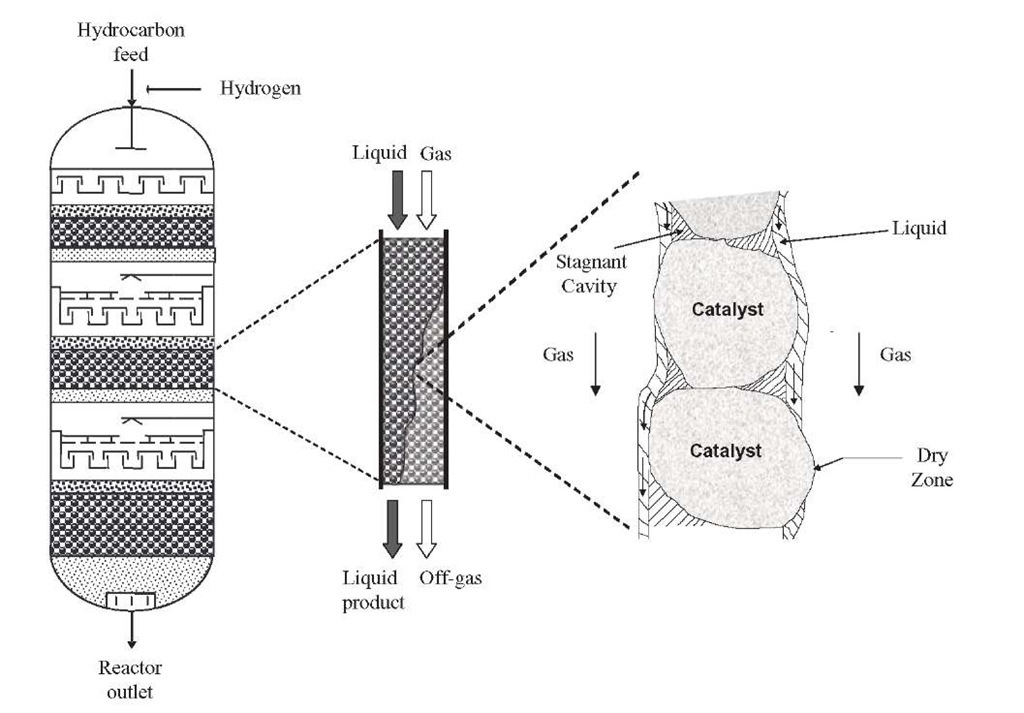
In a TBR the catalyst bed is fixed (Figure 2.1), the flow pattern is much closer to plug flow, and the ratio of liquid to solid catalyst is small. If heat effects are substantial [i.e., highly exothermic reactions such as those occurring in hydrotreating of unsaturated feeds (light cycle oil from fluid catalytic cracking units)], they can be controlled by recycling of the liquid product stream, although this may not be practical if the product is not relatively stable under reaction conditions or if very high conversion is desired, as in HDS, since recycling causes the system to approach the behavior of a continuous-stirred-tank reactor (CSTR). For such high-temperature increases, the preferred solution is quenching with hydrogen, although the use of other streams has also been reported.Even when a completely vapor-phase reaction in a fixed catalyst bed may be technically feasible, a TBR may be preferred to save energy costs due to reactant vaporization. The limiting reactant may be essentially all in the liquid phase or in both the liquid and gas phases, and the distribution of reactant and products between the gas and liquid phases may vary with conversion.
TBR with Co-current Gas-Liquid Downflow A TBR consists of a column that may be very high (above 10 to 30 m), equipped with one or various fixed beds of solid catalysts, throughout which gas and liquid move in co-current downflow. Figure 2.3 shows the typical film flow texture found during a trickle-flow regime (Gianetto and Specchia, 1992). In this mode, gas is the continuous phase and liquid holdup is lower. This operation is the one most used in practice, since there are less severe limitations in throughput than in countercur-rent operation.
For gas-limited reactions (high liquid reactant flux to the catalyst particle, low gas reactant flux to the particle), especially at partially wetted conditions, a downflow reactor is preferred, as it facilitates transport of the gaseous reactant to the catalyst (Dudukovic et al., 2002). In contrast to commercial TBR, in the case of bench-scale TBR operating at equivalent space velocity, the liquid velocity and the catalyst bed length have important effects on the performance of the reactor. The principal advantages and disadvantages of TBR with downflow co-current operation are given below.
Advantages
• Recommended for gas-limited reactions
• Liquid flow approaches plug-flow behavior, which leads to high conversions
Figure 2.3. Nonideal TBR suffering from liquid maldistribution.
• Low liquid-solid volume ratio: fewer occurrences of homogeneous side reactions
• Possibility of varying the liquid rate according to catalyst wetting and heat and mass transfer resistances
• A variety of flow regimes allowed; most flexible with respect to varying throughput demands
• The down flow mode also helps keep the bed in place, although with catalysts that are soft or deformable, this might hasten undesired cementation
• Compared with countercurrent flow operation, for co-current flow of the two phases, no limitation on the throughput arises from the phenomenon of flooding, and the quantities of the phase that can be passed depend only on the upstream pressure available because of vaporization effects
• At higher gas loadings, the texture of the liquid is modified by gas-phase friction, the liquid distribution is improved (lower liquid wall flow), and the pressure drop rises (less rapidly in co-current than in countercurrent flow)
• Easy operation with fixed adiabatic beds; for exothermic reaction systems, gas or liquid streams as quench, and the liquid and/or gas recycle limit temperature rises
• Possibility of operating at higher pressure and temperature
• Pressure drop through the bed is relatively low, thus reducing pumping costs
• Larger reactor size, and generally of simple construction, as there are no moving parts
• Lower investment and operating costs, and low catalyst loss, which is important when costly catalysts are used
Disadvantages
• Limitations on the use of viscous or foaming liquids
• Limited to reasonably fast reactions
• Lower catalyst effectiveness, due to the use of large catalyst particle size
• Particle size cannot be smaller than 1 mm because of pressure drop; risk of increasing pressure drop or obstructing catalyst pores when side reactions lead to fouling products
• Reactor-scale maldistribution, channeling, and incomplete and/or ineffective external catalyst wetting (poor contacting effectiveness) can occur with low liquid flow rates and reactor diameter/particle size ratios (<25)
• Sensitivity to thermal effects, although this drawback can be limited by recycling part of the outlet liquid or injecting cooled gas (quenching)
• Difficulties in the recovery of reaction heat
• Lower liquid holdup compared with co-current gas-liquid upflow
• Deactivation of the catalyst by deposits
• Dismantling of the reactor during catalyst replacement
• In hydrotreating (HDT) reactors, most of the bed is under the H2S and NH3 reach regime and its inhibiting effect is strongest in the region where the refractory sulfur compounds have to be converted. NH3, particularly, strongly suppresses the activity of the acidic function of the hydrocrack-ing catalyst
• H2 partial pressure will be lowest at the HDT reactor outlet due to the combined effect of pressure drop, hydrogen consumption, and reduction of hydrogen purity as gaseous by-product yields (H-S, NH3 – and H-O) increase along the reactor
• Used in downward mode in the refining industry with less conversion; the inhibition effect of H2S and NH3 on the catalyst results in a poorer performance
TBR with Countercurrent Gas-Liquid Flow TBRs operating in countercurrent gas-liquid flow (Figure 2.1) provide an opportunity for selective removal of by-products that may act as inhibitors (e.g., in hydrodesulfurization, where hydrogen sulfide may have an inhibitory effect). The introduction of FBRs with countercurrent flow in a number of refining operations is probably either via redesign of existing reactors or by introduction of new technology. As mentioned earlier, the goal is not an improvement in reactant (hydrogen) mass transfer, which is not rate limiting, but enhanced removal of inhibitory byproducts or in situ product separation. That is why countercurrent flow will become more prominent in the future for processes that suffer from byproduct catalyst inhibition (Dudukovic et al., 2002).
A catalytic PBR with countercurrent mode is a suitable alternative to TBRs for reactions conducted over catalysts with a very large surface area-to-volume ratio. However, the main problem of the countercurrent reactor for commercial application is due to hardware limitations. There is therefore a need to develop improved hardware configurations that allow countercurrent contacting of gas and liquid in the presence of small catalyst particles (Kundu et al., 2003). The main advantages and disadvantages of TBRs with countercurrent flow are given below.
Advantages
• Countercurrent operation is preferred over co – current when a large heat of reaction is involved
• Countercurrent operation gives a more favorable flat axial temperature profile
• Large surface area for vapor-liquid mass transfer
• High ratio of number of active sites to reactor volume
• Easy catalyst handling
• For the HDT process, the major part of the bed is in an H2S-lean regime, which protects from inhibition by H2S formed in a large part of the bed.
• H2 partial pressure is highest at the end of the bed, and temperature in this part can be lowered and more active, less sulfur-tolerant catalysts can be used in the downstream part of the bed, which will favor the chemical equilibrium for reversible reactions [i.e., hydrodearomatization (HDA) reaction]. The effect of equilibrium-limited conversion and product inhibition is reduced
• The major part of the bed is in the NH3 [a by-product of hydrodenitro-genation (HDN) reaction]-lean regime, which favors the HDT reaction by protection from the inhibition of NH3 and H 2S. This operation has great advantages through omitting two separate reactor stages
• The concentration of gas impurities formed during reaction is less in most parts of the bed. This favors the conversion of reactions normally limited by chemical equilibrium and enables handling more difficult feedstocks to obtain higher levels of conversion. Figure 2.4 shows typical partial pressure profiles of H2S along the bed length for co-current and countercurrent operations during hydroprocessing, in which the aforementioned behavior is clearly observed
Figure 2.4. Profiles of H2S partial pressure along the catalytic bed in an HDT reactor (—, co-current; —, countercurrent).
• Countercurrent operation provides the highest hydrogen purity in that part of the bed where the least reactive compounds need to be converted
Disadvantages
• Presence of flooding at high liquid throughputs
• Estimation of liquid holdup, pressure drop, and mass transfer coefficients is difficult since correlations employed to calculate these parameters do not include data for the small porous catalyst packing typically used in PBRs with two-phase flow
• Limited to low velocities far below those of industrial interest, due to the occurrence of excessive pressure drop and flooding problems
• It is not possible to use smaller (1 to 5 mm) catalyst particles than those used in co-current downflow TBRs
• High axial dispersion effects in the liquid phase
Packed Bubble-Flow Reactors with Co-current Gas-Liquid Upflow This classification includes upflow reactors, upflow co-current reactors, packed-bubble columns, upflow packed-bubble columns, and flooded fixed – bed reactors. In bubble-flow operation a continuous liquid phase, together with a dispersed gas phase, move upward co-currently through the packed bed (Figure 2.1). Such an operation would be recommended in cases where liquid reactants are treated with a relatively small amount of gas, as in the hydration of nitro compounds and olefins, or where a relatively large liquid residence time is required for the degree of conversion desired. Use of these reactors assures complete external wetting of the catalyst and high liquid holdup. In this mode the liquid is typically the continuous phase.
Bubble operation is also advantageous when the reactor diameter/particle diameter ratio is relatively small, because the liquid catalyst contact is more effective than in trickle operation. Compared with empty bubble columns, the packed bed has the advantage of reducing substantially backmixing in the flowing phases as well as the coalescence of gas bubbles. Under any conditions the wall heat transfer coefficient should also be higher than it is in trickle operation (Hofmann, 1978).
For liquid-limited reactions (low liquid reactant flux to the catalyst particle, high gas reactant flux to the particle), an upflow reactor should be preferred, as it provides complete catalyst wetting and the fastest transport of the liquid reactant to the catalyst. For very shallow catalyst beds, upflow operation gives much better conversions than downflow operation under the same reaction conditions. The gas and liquid flow rates typically used in a bench-scale down-flow trickle-bed HDS reactor are such that when they are used in co-current upflow operation, a bubble flow regime will be generated.
The performance of a reactor under this hydrodynamic flow condition should be considerably different from the one obtained under trickle- flow conditions. In an upflow system the low-boiling components, which are generally more reactive, pass into the vapor phase and are swept out more rapidly than the high-boiling material, which progresses relatively slowly through the bed. This superior performance of upflow processing is attributed to the long residence time of the heavy liquid fractions, but a more important factor may be the very low liquid flow used (Satterfield, 1975).
When both gas and liquid flow upward, maldistribution of liquid or incomplete catalyst wetting should not be very important, particularly when the hydrodynamic conditions of bubble flow prevail within the reactor. An upflow (flooded bed) reactor, which should give good solid–iquid contacting, could be used instead of an autoclave to obtain information on the intrinsic kinetics. The main advantages and disadvantages of TBRs with co-current upflow are given below.
Advantages
• Recommended for liquid-limited reactions
• Liquid holdup is higher. The liquid holdup is larger in an upflow operation than in a downflow operation under similar conditions
• Better effective wetting
• Better thermal stability for highly exothermic reactions
• High liquid saturation
• The liquid flow can be more uniformly distributed (better distribution of liquid throughout the catalyst bed)
• The gas-liquid and liquid-solid mass transfer coefficients are larger in an upflow operation than in a downflow operation
• In backmix flow conditions, where variations in gas and liquid flow rates change the conversion, upflow operation gives better results than down-flow operation under the same conditions
• Larger effective residence time
• If a catalyst gradually becomes deactivated by the deposit of polymeric or tarry materials, the upflow reactor may maintain its activity longer by washing off these deposits more effectively
• For rapid and highly exothermic reactions, heat transfer between liquid and solid may also be more effective in upflow than in downflow operation
Disadvantages
• For HDT operations, conversions of sulfur, metals, and asphaltenes decrease with an increase in gas and liquid flow rates at constant temperature and pressure. Conversion of sulfur in upflow operation is reduced faster with time than in downflow operation; however, the conversion is always highest
• Higher pump requirements in order to overcome the hydrostatic head of the liquid
• The need of some designs to avoid the fluidization of the catalyst unless the catalyst was held in place by an extra weight or suitable mechanical methods
• If limiting reactant is present in both phases, over a range of operating conditions in which catalyst pellets filled with liquid are diffusion limited, an upflow reactor would be expected to exhibit a lower reaction rate than a partially wetted TBR
• Formation of stagnant zones inside the catalyst bed
• Higher axial dispersion compared with the downflow mode of operation
Slurry-Bed Reactors
The best alternative to the use of a fixed – bed reactor with two – phase flow, either upward or downward, is a slurry-bed or ebullating-bed reactor in which the catalyst particles, which must be substantially smaller, are in motion. These reactors are sometimes termed three- phase fluidized – bed reactors or suspended-bed reactors (Figure 2.1). The main advantages and disadvantages of slurry-bed reactors are given below.
Advantages
• High heat capacity to provide good temperature control
• Potentially high reaction rate per unit volume of reactor if the catalyst is highly active
• Easy heat recovery
• Adaptability to either batch or flow processing
• Much lower pressure drop
• The catalyst may readily be removed and replaced if its working life is relatively short
• Continuous removal of solid material formed in reaction
• Because of high intraparticle diffusion rate, small particles can be used, which may allow for operating at catalyst effectiveness factors approaching unity, of special importance if diffusion limitations cause rapid catalyst degradation or poorer selectivity
• Lower external mass transfer resistance by means of a high stirring speed
Disadvantages
• Residence-time distribution patterns are close to those of a CSTR, which makes it difficult to obtain high degrees of conversion except by staging and/or increasing operation temperature
• Generation of fine particles by abrasion of the catalyst
• Catalyst removal by filtration may provoke problems with possible plugging difficulties on filters, further time of operation, and the costs of filtering systems may be a substantial portion of the capital investment
• Higher catalyst consumption than that of fixed-bed reactors
• Difficult to scale up
• The high liquid-to-solid ratio in a slurry-bed reactor allows homogeneous side reactions to become more important, if any are possible
• Potential hazard of localized overheating in the reactor because of bad fluidization
• Backmixed flow and the volume of the reactor are not fully utilized