Introduction
Although the revised 2008 World Health Organization (WHO) criteria (Swerdlow et al. 2008) for the diagnosis and classification of Philadelphia chromosome-negative chronic mye-loproliferative neoplasms (MPNs) was defined by a panel of expert hematopathologists and clinicians,serious concern has been repeatedly expressed for its emphasis upon specific histological bone marrow (BM) features (Spivak and Silver 2008). On the other hand, contemporary diagnostic approach regards BM morphology as an integral part, in context with clinical findings and genetics.Moreover, it should not be overlooked that preceding the original descriptions of BM characteristics in MPNs by the WHO (Jaffe et al. 2001), a number of authors had already endorsed some important issues that later were incorporated into this classification (Buhr et al. 1992, 1993; Dickstein and Vardiman 1993; Georgii et al. 1998; Kvasnicka et al. 1997; Thiele et al. 1989a, b, 1996, 1999). On the other hand, the majority of clinical trials usually followed the postulates of the Poly-cythemia Vera Study Group (PVSG) or related diagnostic guidelines (Barosi 1999; Murphy 1999; Pearson 1998; Wasserman 1986). This review tries to highlight not only histological BM features characterizing the main MPNs like poly-cythemia vera, essential thrombocythemia, and primary myelofibrosis according to the updated WHO criteria (Vardiman et al. 2009) but also more controversial issues like standardization as well as reproducibility of BM morphology that are significantly associated with accurate diagnosis.
Polycythemia Vera (PV)
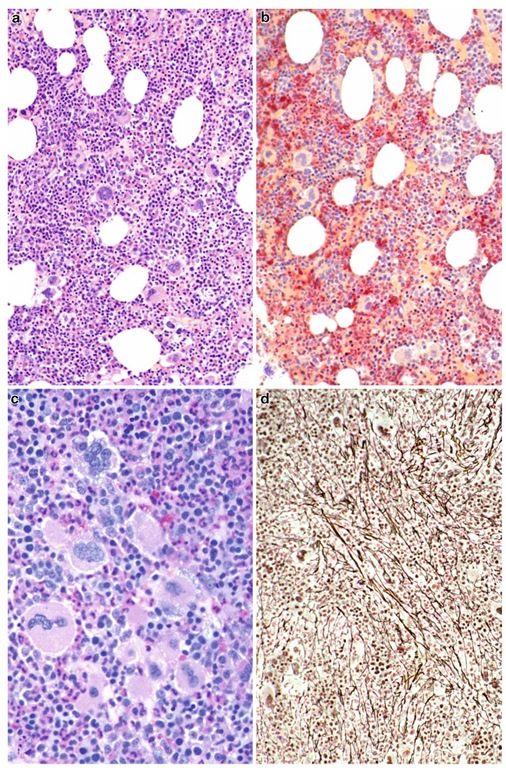
Usually, in PV, hypercellularity of the BM is a characteristic feature (Dickstein and Vardiman 1993; Georgii et al. 1998; Thiele et al. 2001a, 2005a) due to a trilineage proliferation (so-called panmyelosis) involving nucleated erythroid precursors, megakaryocytes, as well as neutrophil granulopoiesis (Fig. 3.1a). Contrasting this finding in BM trephines described in the PVSG trial, there was a wide variation among individuals with about 13% of the cases showing a normal cellu-larity (Ellis and Peterson 1979; Ellis et al. 1986). In this context, one should be aware that in the elderly population of patients presenting with PV, the subcortical BM spaces are regularly transformed to adipose tissue, and any expansion of hematopoiesis toward this area implies hypercel-lularity when evaluated in a representative core biopsy performed at an orthograde direction (Thiele and Kvasnicka 2005a; Thiele et al. 2005c). Quantification of erythropoiesis in relation to neutrophil granulopoiesis reveals a clear shifting to the red cell lineage including many precursors (Fig. 3.1b). Particularly, the normally small and rounded islets of nucleated erythroid precursors display not only a conspicuous enlargement but also a tendency for merging into sheets (Fig. 3.1b). Megakaryocytes have been acknowledged to exhibit peculiar features that facilitate a distinction between PV and reactive erythrocytosis (Georgii et al. 1998; Thiele et al. 2001a). It is noteworthy that this lineage is characterized by a polymorphous aspect showing mature mega-karyocytes of very different sizes, i.e., small- to medium-sized and large to giant forms are either dispersed or loosely clustered (Fig. 3.1a, c). All these findings are a significant extension to former descriptions by the PVSG in which 95% of the BM specimens demonstrated only an increase in megakaryocyte numbers (Ellis and Peterson 1979). A minor to moderate increase in reticulin fibers (Fig. 3.1d) has been reported to be seen already at presentation in 10-20% of patients (Ellis and Peterson 1979; Georgii et al. 1998; Thiele et al. 2006). Until now, a conflict of opinion exists whether this feature is consistent with a later diagnosis and/or a more aggressive course of disease progressing into post-PV myelofibrosis (Barosi et al. 2008; Ellis et al. 1986). Discrimination (relative frequency and ranking) of standardized BM features in PV versus reactive erythrocytosis (Thiele and Kvasnicka 2005a) and the other MPN entities as well (Thiele et al. 2005a) resulted in a substantial prediction of group membership concerning correct diagnosis.
Concerning the endpoints in the evolution of the disease process in PV, the 2008 WHO classification recognizes explicitly a prodromal, so-called prepolycythemic stage,that initially fails to present with a significant increase in the red cell mass or hemoglobin/hema-tocrit level (Kvasnicka and Thiele 2010; Ruggeri et al. 2003) ; Consequently, these cases are not conforming with the original or updated diagnostic criteria of the PVSG (Murphy 1999; Pearson 1998; Wasserman 1986). Histopathology of the BM displays some overlapping features to essential thrombocythemia (ET), especially concerning the prevalence of large to giant hyperlobulated megakaryocytes in addition to an only moderate increase in cellularity including erythropoiesis (Gianelli et al. 2008; Thiele et al. 2005b). For this reason, at onset differentiation from ET may be difficult, and further clinical and JAK2 mutation status investigations are needed (Gianelli et al. 2008; Kvasnicka and Thiele 2010).
The terminal endpoint is the so-called spent phase/postpolycythemic myeloid metaplasia according to the PVSG (Ellis et al. 1986; Murphy 1999) consistent with post-PV myelofibrosis (Barosi et al. 2008) with BM features revealing conspicuous changes. Contrasting manifest poly-cythemic PV, there is a prominence of a left-shifted neutrophil granulopoiesis associated with a reduction of erythroid precursors and an increase of a dense meshwork of reticulin fibers intermingled with collagen (Buhr et al. 2003; Ellis et al. 1986; Georgii et al. 1998; Thiele and are increased and may be clustered, but fail to show maturation defects (compare with Fig. 3.3c). (d) Moderate increase in reticulin may be found in a number of patients already at diagnosis. Magnification: a, b, d, x180; c, x380. Staining: a=hematoxylin and eosin; b=AS-D-chloroacetate esterase reaction; c=periodic acid Schiff reagent (PAS); d=silver impregnation after Gomori Kvasnicka 2005a). Overt collagen fibrosis resembling phenotypically primary myelofibrosis (PMF) is usually considered as the forerunner of myelodysplastic and leukemic transformation showing either severe maturation defects of cell lineages or more than 20% blasts (Thiele and Kvasnicka 2005a; Thiele et al. 2006; Vardiman et al. 2009).
Fig. 3.1 Polycythemia vera. (a) Conspicuous hypercellu-larity for age with increase of all three cell Lineages (pan-myelosis) including extended islets of nucleated erythroid precursors and dispersed mature megakaryocytes. (b) Prominent enlargement of erythropoiesis surrounded by left-shifted neutrophil granulopoiesis (red). (c) Megaka-ryocytes of different size ranging from small to giant ones
Essential Thrombocythemia (ET)
In BM biopsy specimens derived from ET patients, usually neither a relevant increase in overall cel-lularity (Fig. 3.2a) nor a significant proliferation of a left-shifted neutrophil granulo- or erythropoi-esis is detectable (Fig. 3.2b). The majority of cases present with an inconspicuous appearance of these cell lineages. On the other hand, randomly distributed or loosely clustered large to giant mature megakaryocytes with deeply folded nuclei (so-called staghorn type) surrounded by correspondingly mature cytoplasm (Fig. 3.2c) are the outstanding features (Buhr et al. 1992; Florena et al. 2004; Georgii et al. 1998; Gianelli et al. 2006; Thiele et al. 1989a, 2005d; Thiele and Kvasnicka 2006b). Usually, there is no increase in BM fibers (Fig. 3.2d). Only in a small subfraction of patients (about 5%) minor, i.e., grade 1, reticu-lin fibrosis (Thiele et al. 2005c) according to WHO may be observed.Until now, it is not clear if this feature may be associated with a later diagnosis/performance of the biopsy in this very small cohort of patients. According to the WHO criteria any substantial increase in reticulin or occurrence of collagen fibers is not compatible with ET at onset contrasting terminal stages of disease (Barosi et al. 2008). Presence of BM fibrosis as well as proliferation of neutrophil granulopoiesis and abnormalities of the mega-karyocytic cell lineage raises the possibility of PMF (Gianelli et al. 2006; Thiele et al. 2000, 2003). It has to be realized that significant differences are encountered in the diagnosis of ET when following the guidelines of the PVSG (Murphy et al. 1997; compared to those of the WHO (Thiele and Kvasnicka 2003b) . Because the PVSG unfortunately failed to render a detailed description of BM features characterizing ET on the one hand, but allows a certain amount of fibro-sis among their diagnostic parameters on the other hand (Murphy et al. 1997; Murphy 1999; Pearson 1998), discrimination from early/prefibrotic stages of PMF with presenting thrombocythemia (so-called false ET) is not feasible (Florena et al. 2004; Gianelli et al. 2006; Thiele et al. 1989b, 2000, 2011; Thiele and Kvasnicka 2003b, 2006b). A clear-cut differentiation between ET and early/ prefibrotic PMF is an essential issue for the outcome including progression to overt myelofibro-sis, transformation to leukemia, as well as survival.Further differentiation includes initial/early stages of PV clinically presenting with overt thrombocythemia and therefore mimicking ET (Gianelli et al. 2006; Thiele et al. 2005b). In these cases, BM shows a mild hypercellularity due to trilineage proliferation (panmyelosis) including large to giant mega-karyocytes with hyperlobulated nuclei (Kvasnicka and Thiele 2010). Transformation of WHO-diagnosed ET into acute leukemia (AML) is a rare event with a cumulative incidence of 0.7% at 10 years,compared to the reported incidence of 8.3% when following the PVSG criteria (Girodon et al. 2010). Consequently, frequency of AML transformation occurred significantly less (range 1.4-3.0%) when investigating cohorts that included also fractions of WHO-diagnosed ET patients (Palandri et al. 2009; Passamonti et al. 2008; Wolanskyj et al. 2006) . Similarly, risk of post-ET myelofibrosis (Barosi et al. 2008) is also significantly dependent on the applied diagnostic criteria. Following the PVSG guidelines (Murphy et al. 1997), the 10-year risk was found to range from 8.3% to 9.7% (Cervantes et al. 2002; Chim et al. 2005), whereas the WHO-classified ET patients revealed a corresponding rate of only 0.8%.Again, series that consisted of a mixture of either PVSG- or WHO-defined ET patients revealed a higher incidence of myelofibrosis ranging between 3.8% and 4.9% (Palandri et al. 2009; Passamonti et al. 2008; Wolanskyj et al. 2006).
Fig. 3.2 Essential thrombocythemia. (a) Normal age-matched cellularity, except for a prominent increase in large to giant megakaryocytes loosely clustered or dispersed throughout the bone marrow space. (b) No proliferation or significant left shift of neutrophil granulopoiesis or erythropoiesis surrounding giant mature megakaryo-cytes. (c) Conspicuous increase in large megakaryocytes without maturation defects showing hyperlobulated, occasionally staghorn-like nuclei. (d) No increase in reticulin fibers, but giant megakaryocytes with deep foldings of their nuclei. Magnification: a, b, c, x180; d, x380. Staining: a = hematoxylin and eosin; b = AS-D-chloro-acetate esterase reaction; c = periodic acid Schiff reagent (PAS); d = silver impregnation after Gomori
This impact of BM morphology and accurate classification on complications and outcome of ET is reflected by corresponding data on survival.
Primary Myelofibrosis (PMF)
Concerning BM morphology in PMF in large series clinicians have reported a striking variability in hematological findings at the time of first presentation.This conspicuously wide spectrum of clinical manifestations is paralleled by BM features that in the beginning may show a hypercellularity with no or only minor increase in reticulin fibers or, in advanced stages, specimens presenting with a hypocellular marrow and overt collagen fibrosis and osteosclerosis (Buhr et al. 2003; Dickstein and Vardiman 1995; Kvasnicka et al. 1997; Thiele et al. 1989a, 2003; Thiele and Kvasnicka 2005b, 2006a) consistent with classical myelofibrosis with myeloid metaplasia (Barosi 1999; Cervantes and Barosi 2005). The concept of an early stage of PMF characterized among others by the BM fiber content raises the salient question of progression or a stepwise evolution of the disease process. This important issue has been settled by scrutinized clinicopathologi-cal follow-up studies involving sequential BM biopsy specimens with grading of myelofibrosis and reference to hematological findings (Buhr et al. 2003; Kreft et al. 2005; Thiele et al. 2003; Thiele and Kvasnicka 2006a) . Consequently, this finding of developing myelofibrosis and accompanying clinical presentations was adopted by the WHO classification.
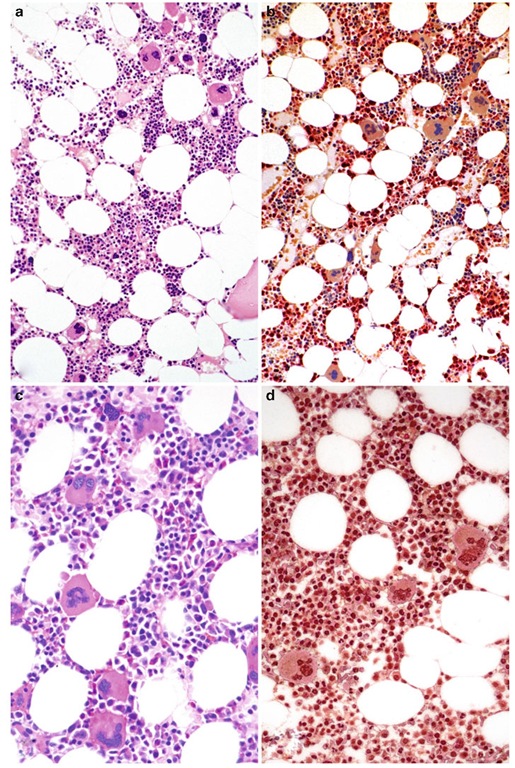
In the early/prefibrotic stage at the initial endpoint of disease evolution, histopathology of the BM is characterized by hypercellularity (Fig. 3.3a) consisting of a prominent neutrophil granulocytic and megakaryocytic proliferation (Fig. 3.3b) which is often associated with a slight to moderate reduction of nucleated red cell precursors (Buhr et al. 2003 ; Florena et al. 2004; Gianelli et al. 2006; Kreft et al. 2005; Thiele et al. 2001b, 2003; Thiele and Kvasnicka 2004, 2005b). Most important in this setting are conspicuous abnormalities of the megakaryocytic cell lineage (Fig. 3.3a, c, d) . These include megakaryocyte arrangement and localization in the marrow space (histotopography with endosteal-paratrabecular dislocation, forming of dense or loose clusters) and a high variability in size (small and giant forms) (Fig. 3.3a). Moreover, there are significant aberrations of nuclear organization (marked hyp-olobulation, irregular folding, condensed chro-matin patterns) generating bulbous or so-called cloud-like/balloon-shaped nuclei, increased nuclear-cytoplasmic ratio (maturation defect), as well as increased numbers of bare (denuded) nuclei (Fig. 3.3c). At this so-called hypercellular early stage of PMF, there is no or only grade 1 reticulin fibrosis (Thiele and Kvasnicka 2005b) detectable (Fig. 3.3d), and because of the remarkable mega-karyocyte proliferation, differentiation from ET is a key issue (Buhr et al. 1992; Florena et al. 2004; Gianelli et al. 2006; Thiele et al. 2000, 2001b; Thiele and Kvasnicka 2003a, b).
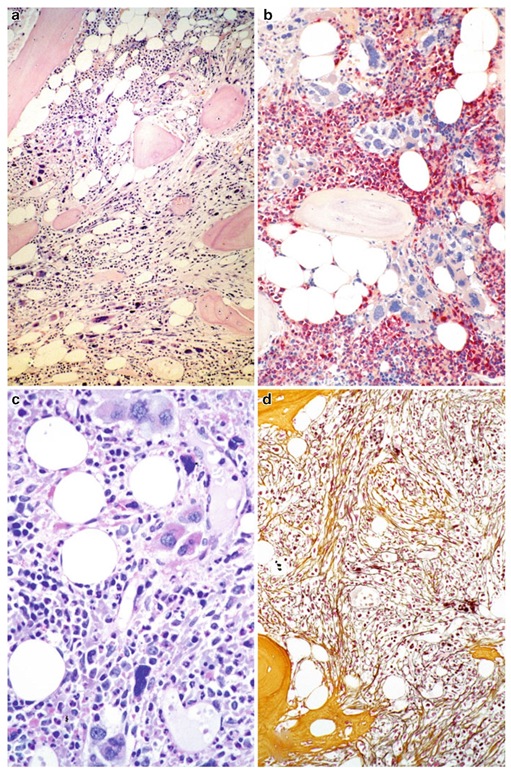
The other, well-known , i.e., significantly advanced to terminal endpoints characterizing the evolution of PMF are represented by the classical clinical features of myelofibrosis with myeloid metaplasia .These were renamed overt fibrotic PMF.Contrasting the early stages, BM cellular-ity is variable with areas of patchy hematopoiesis that may be separated by adipose tissue or gross fibrosis causing a streaming effect (Fig. 3.4a). Although neutrophil granulopoiesis as well as erythropoiesis are usually reduced in the overall hypocellular BM, there may still be some areas of residual hematopoiesis detectable (Fig. 3.4b). Similar to the early stages but often more pronounced abnormalities are prevalent in the clustered megakaryocyte (Fig. 3.4c) that together with precursors of the other cell lineages are often localized along as well as in the dilated marrow sinuses. A grossly fibrotic BM matrix with a dense increase in reticulin and collagen conforming with grades 2 and 3 myelofibrosis (Thiele et al. 2005c) is recognizable (Fig. 3.4d), often associated with osteosclerosis, i.e., new bone formation (Buhr et al. 1993, 2003; Dickstein and Vardiman 1993; Georgii et al. 1998; Thiele et al. 2003, 2005d, 2006; Thiele and Kvasnicka 2005b). In advanced or terminal PMF, the BM is not only characterized by an overt reticulin and collagen myelofibrosis but also by a dramatic alteration concerning the vascular architecture including frequency and shape of the vessels. Contrasting the early/prefibrotic stages without, or minor, reticulin following the progression of fibrosis, a significant increase in quantity of the microvas-culature is noticeably associated with marked luminal dilatation and tortuosity (Kvasnicka and Thiele 2004; Thiele et al. 1992). Although restricted to overt (classical) PMF or post-ET and post-PV myelofibrosis in comparison with other MPN entities, ample evidence has been produced regarding the significant enhancement of vascular proliferation (Arora et al. 2004; Boveri et al. 2008; Lundberg et al. 2000; Mesa et al. 2000; Ni et al. 2006). These remarkable changes were assumed to be in keeping with a complex functional network existing between fibrillo- and neoangiogenesis (Boiocchi et al. 2011; Boveri et al. 2008; Ni et al. 2006). In terminal stages, the BM may be almost totally effaced by collagen fibrosis associated with pronounced osteosclerosis and/or increased dysplastic changes of hematopoi-etic cells and blastic transformation, clinically causing BM failure and AML.
Fig. 3.3 Early/prefibrotic primary myelofibrosis. (a) Remarkable hypercellularity for advanced age revealing large clusters of megakaryocytes abnormally dislocated toward the trabecular bone. (b) In addition to megakaryo-cyte proliferation, there is a conspicuous increase in neu-trophil granulopoiesis (red) accompanied by reduction of nucleated erythroid precursors. (c) Clustered small to giant megakaryocytes show a prevalence of striking maturation defects including cloud-like, hypolobulated, and hyper-chromatic nuclei with only some irregular foldings. (d) Only minimal increase in reticulin fibers. Magnification: a, b, d, x180; c, x380. Staining: a=hematoxylin and eosin; b=AS-D-chloroacetate esterase reaction; c = periodic acid Schiff reagent (PAS); d = silver impregnation after Gomori
Fig. 3.4 Advanced primary myelofibrosis. (a) Reduced cellularity with streaming-like pattern of residual hematopoiesis including atypical megakaryocytes in addition to initial osteosclerosis (new bone formation). (b) In other areas, there may be still a patchy hematopoiesis with densely clustered megakaryocytes surrounded by neutro-phil granulopoiesis (red) but only a very few erythrocyte precursors. (c) Clustered megakaryocytes are lying along a dilated sinus and reveal severe aberrations of maturation including cloud-like, dense, or abnormally lobulated nuclei. (d) Overt myelofibrosis showing bundles of collagen (yellow; between a dense network of reticulin and osteosclerosis. Magnification: a, b, d, x180; c, x380. Staining: a=hematoxylin and eosin; b=AS-D-chloroacetate esterase reaction; c = periodic acid Schiff reagent (PAS); d = silver impregnation after Gomori