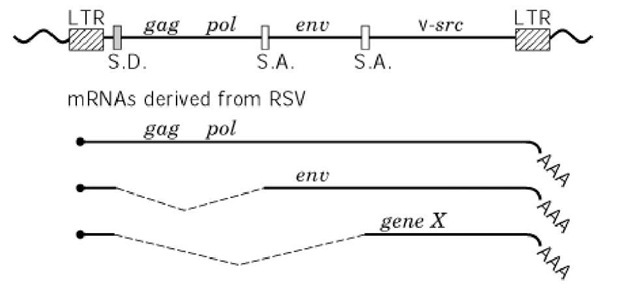
Rous sarcoma virus (RSV) was discovered in the extract of chicken sarcoma by Dr. P. Rous in 1911 as the first infectious reagent that causes tumors, and it is now known to be the prototype oncogenic retrovirus (Oncovirinae). It is widely accepted that ALV (avian leukosis virus), a replication-competent retrovirus carrying the genes gag (internal structural proteins), pol (reverse transcriptase and DNA endonuclease) (see HIV (Human Immunodeficiency Virus) and Hepatitis B Virus), and env (envelope glycoproteins), had once picked up a gene present in the chicken chromosome, now designated as cellular src (c-src), during its replication cycle as a rare event. RSV therefore carries an extra gene designated viral src (v-src) (because it is derived from c-src) at the extreme 3 region of the genome, and it can be expressed from a subgenomic messenger RNA produced by RNA splicing (Fig. 1). By changing from c-src upon its integration into the virus genome and by accumulation of mutations during the subsequent propagation of virus, this virus gene had acquired the ability to cause neoplastic transformation in chickens. High-level expression of c-src, however, does not cause such transformation. The products of both the v-src and c-src genes are now known to be tyrosine-kinases, with molecular weights of 60 kDa, and p60v-src has much higher kinase activity than does p60c-src
Figure 1. Proviral DNA structure of Rous sarcoma virus. The line at the top indicates the chromosomal DNA containing proviral DNA. SD, splicing donor site; and SA, splicing acceptor site. Three transcripts are produced from the LTR promoter, as shown below.
This prototype virus, however, is exceptional among RNA tumor viruses in that it is replication-competent. All the other oncogenic retroviruses acquired proto-oncogenes at the expense of a part of their genomes that is essential for virus replication, and they therefore require the association of other replication-competent virus such as ALV (often called helper virus) for their propagation. Because of the advantage of this exceptional genome structure, RSV has been historically used in laboratory for the analysis of neoplastic transformation in cell culture; virus titers, which were originally assayed by sarcoma formation in the injected chicken, are now determined much more easily by formation of a focus (a group of transformed cells) in infected chicken embryo fibroblasts (CEFs). RSV-infected CEF assume refractile morphology and acquire the ability to grow in soft agar (anchorage-independent growth). These phenotypes unique to cellular transformation are maintained even after cell divisions, because the virus genome is also replicated as a proviral DNA that is integrated into the host chromosome.
One of the breakthroughs in oncogene research was the isolation of unique temperature-sensitive mutants of RSV. These mutant RSVs were replication-competent independent of the temperature, but the transformation phenotypes of the infected cells were temperature-dependent. At 37°C, infected cells assume fully transformed morphology, while cells become flatter and assume very similar morphology to that of uninfected CEF at 40°C to 41°C. These observations led to the discovery of oncogenes and revealed the following basic ideas about them:
1. RSV encodes a gene responsible for the initiation of cellular transformation (the oncogene now known to be v-src, described above).
2. The oncogene is not necessary for the viral replication.
3. Continuous function of the oncogene is necessary for the maintenance of cellular transformation. (This observation excluded another hypothesis, the "hit and run theory," in which the virus infection process is supposed to cause irreversible phenotypic changes to the host cells.)
It is also noteworthy that RSV-based avian retrovirus vectors are now widely used as a powerful tool for basic research. By transfecting plasmid DNA carrying a proviral DNA in which the v-src sequence was substituted with an exogenous gene, replication-competent virus vectors expressing the exogenous gene can be recovered from the culture fluid of the transfectants and used to introduce foreign genes into several avian primary cultures, embryos, or adults quite efficiently.