Phosphofructokinase, or PFK (EC 2.7.1.11), is one of the best characterized allosteric enzymes. It catalyzes the step of glycolysis in which fructose-6-phosphate (FRU-6P) is phosphorylated to fructose-1,6-bisphosphate (FRU-1,6P2) using either ATP or pyrophosphate (PPj) as a phosphatedonor. This enzyme is present in most types of eukaryotes and eubacteria but occurs rarely in archaebacteria. PFKs from animals, yeast, and most bacteria use ATP as a phosphate donor. Those that use PPj are found in plants, protozoa, and some bacteria, often in addition to ATP-dependentenzymes. The amino acid sequences of most of the ATP-dependent PFKs are homologous, indicating that they form an evolutionary gene family. This family includes both "small" bacterial enzymes and "large" enzymes from mammals and yeast. It is a good model of enzyme evolution and of the appearance of "new" regulatory functions. PFK in most organisms is a highly regulated enzyme that has several allosteric effectors, and it controls (at least in part) the rate of glycolysis. The PFK from Escherichia coli has become a paradigm for the concerted allosteric model (1). X-ray crystallography, site-directed mutagenesis, and enzyme kinetic studies on bacterial ATP-dependent PFKs have also explained their catalytic and regulatory properties in some detail.
1. Isoenzymes
In humans (and probably in other mammals), there are three homologous types of PFK polypeptide chains, the muscle M, liver L, and platelet P isoenzymes. They are encoded by different genes, located on chromosomes 21, 1, and 10, respectively. All have several introns. The few cases of alternative splicing reported always lead to deletion of one exon and formation of a nonfunctional enzyme. Even though the M, L, and P type proteins are different, they share enough similarities assemble into active, multiple, heterologous oligomers, usually tetramers. The many different PFK isoforms generated in this way have somewhat different catalytic and regulatory properties, such as saturation by ATP and Fru-6P or sensitivity to allosteric effectors (2). Some species, such as E. coli, also have other minor proteins with PFK activity, but have unrelated amino acid sequences indicating that they do not belong to the main family of PFKs.
2. Metabolic Role
The reaction catalyzed by PFK, using either ATP or PPi, is irreversible under physiologicalconditions. Its complex regulation by adenine nucleotides and by various metabolites indicates that PFK controls the production of cellular energy and the carbon flux between glucose and pyruvate. In several instances, the activity of PFK is coupled to that of pyruvate kinase because Fru-6P, the substrate of PFK, is an allosteric activator of one form of pyruvate kinase. This coupling is stronger in bacteria, where phospho enolpyruvate (PEP), the substrate of pyruvate kinase, is the allosteric inhibitor of PFK. In some microorganisms (Gram-positive bacteria and mycoplasms, but not E. coli), the genetic expression of these two enzymes is coordinated. They catalyze reactions seven steps apart in glycolysis. In these cases, the genes that code for PFK and pyruvate kinase are adjacent on the chromosome and constitute an operon under transcriptional control by the same promoter (3).
The enzymatic (and sometimes genetic) coupling between two enzymes that each catalyze irreversible phosphoryl transfer steps is probably crucial for controlling the glycolytic flux, especially in anaerobes that use glycolytic fermentation as their main energy-producing pathway.
3. Primary Structures and Evolution
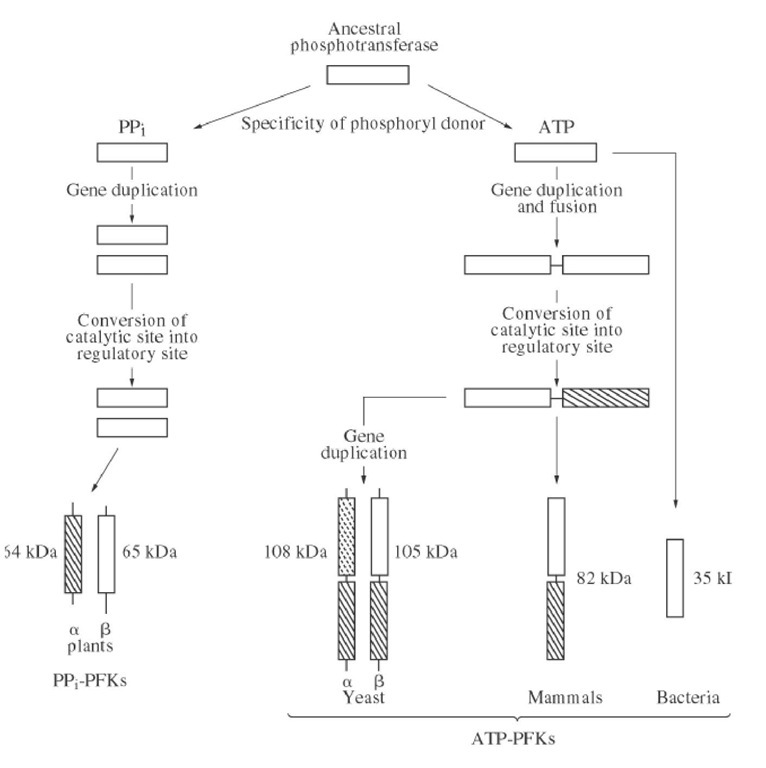
Most of the ATP-dependent PFKs, whether from animals, yeasts, or bacteria, have homologous sequences and belong to an evolutionary gene family. The simplest members of this family are bacterial PFKs whose polypeptide chains are about 35 kDa. Mammalian PFKs are approximately twice as large, about 80 kDa, and have an internal sequence repeat. Their N- and C-terminal halves are homologous and both resemble bacterial PFKs. This suggests that they have been produced by tandem gene duplication and fusion (4). All of the important active site residues are conserved in the N-terminal half, indicating a catalytic function, whereas replacement of several crucial residues, notably that of Asp127 by Ser, suggests that the C-terminal half is inactive. Yeast PFK is composed of two types of related chains, a of 108 kDa and b of 105 kDa. Each has a segment homologous to mammalian PFKs and unrelated N- and C-terminal extensions. These chains have formed by duplication of a mammalian-like gene followed by independent acquisition of terminal extensions (Fig. 1). The distribution of residues crucial for catalysis, plus various kinetic studies, suggest that the active site is carried by the N-terminal half of the b-chain and that the a-chain has only a regulatory role. Homologous residues are referred to here by their numbers in the E. coli enzyme.
Figure 1. Evolution of PFKs suggested by the comparison of their primary sequences. The unrelated segments are represented by thin lines. Among the homologous segments, those that have retained a catalytic function are open, and those that have lost their catalytic abilities and evolved to acquire a regulatory function are crossed. The special weak crossing on the N-terminal segment of the yeast a chain reflects that the evidence in favor of a pure regulatory role is weaker than for the other eukaryotic PFKs.
PPj-dependent PFKs are made of two homologous chains, each of which has a segment distantly related to bacterial PFKs. This suggests that all PFKs might derive from the same ancestral protein (Fig. 1). The conservation of critical residues indicates that the catalytic site is on the b-chain and that the a-chain is regulatory.
4. Quaternary Structure
Bacterial and mammalian PFKs are active as tetramers and are inactivated upon dissociation into dimers (see Quaternary Structure). The allosteric inhibition of the PFK from Thermus thermoaquaticus by PEP involves such a dissociation. The crystal structures of bacterial PFKs explain why an oligomeric state is required for activity. The Fru-6P binding site is at the interface between two subunits. The fructose moiety interacts with one subunit and the 6-phosphate group with the other (5). Under some conditions, tetrameric mammalian PFKs associate further into octamers and even more complex structures. They are active, but the role of this aggregation is not clear. PPj-dependent PFKs also have a tetrameric structure that contains two a- and two b-chains. Yeast PFK is active only as an octamer that has four a- and four b-chains.
5. Catalytic Mechanism of Phosphoryl Transfer
Experiments with chiral (16O, 17O, and 18O)-ATP have shown that transfer of the g-phosphate from ATP to Fru-6P occurs directly within a ternary complex (6) without a phosphoryl-enzyme intermediate. Steady-state kinetics are compatible with a random order of substrate binding, and the pH-dependence of the maximum velocity suggests that Mg -ATP is the most active ionic form (7).
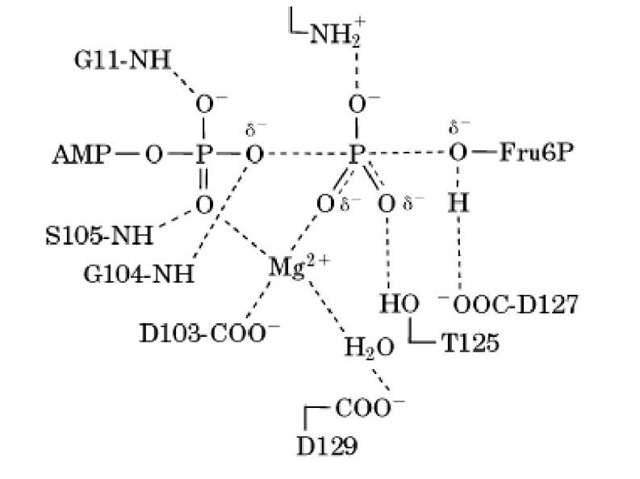
X-ray crystallography of bacterial PFKs and site-directed mutagenesis suggest that several PFK active site groups are involved in catalyzing phosphoryl transfer (Fig. 2). The deprotonated side chain of Asp127 acts as a base and abstracts a proton from the 1-OH group of Fru-6P so as to increase its nucleophilicity. Mutation of Asp127 to Ser decreases the catalytic rate constant 104-fold (8). The transferred phosphoryl group is stabilized by interactions with the magnesium ion, by the positive charge of Arg72, and by a hydrogen bond with Thr125. The deprotonated side chains of Asp103 and Asp129 hold the magnesium ion that contributes electrostatic catalysis. The amide NH of Gly104 makes a hydrogen bond with the bg-oxygen bridge to facilitate cleavage of the O_P bond (5). X-ray crystallography also shows that the active site of E. coli PFK takes two conformations, one "open" that binds substrates and releases products and one "closed" in which catalysis occurs (5). Rapid kinetics have shown that conformational changes indeed occur in E. coli PFK upon substrate binding (and/or product release) and that these changes are slow enough to be rate-limiting in some conditions for the catalytic cycle (9).
Figure 2. The transition state of the reaction catalyzed by PFK with the main interactions within the active site as suggested by X-ray crystallography (5) and site-directed mutagenesis. The residues shown are conserved in all of the active PFKs except for Thr125, replaced by a serine in eukaryotic PFKs, which conserves an OH group. The partial covalent bonds are indicated by broken lines, and the hydrogen and electrostatic bonds by dotted lines.
NADH using aldolase, triosephosphate isomerase, and glycerol-phosphate dehydrogenase (10, 11). 7. Regulation of activity
The catalytic activities of all PFKs are sensitive to pH, ionic strength, divalent metal ions and/or their chelators (the real substrate is the ATP – Mg2+ complex), and phosphate, NH4 13 and K+ ions (10. 11). In addition, PFKs often have complex regulatory properties, such as sensitivity to allosteric effectors and/or a cooperative saturation by the substrate Fru-6P. Only a few (eg, that from Dictyostelium discoideum) follow Michaelis-Menten kinetics under all conditions studied.
7.1. Allosteric Effectors
The activities of PFKs are modulated by several allosteric effectors that differ among various organisms (Table 1). Most of these effectors do not modify the maximum velocity, but affect only the saturation by Fru-6P, influencing the cooperativity and/or half-saturating concentration. All PFKs are activated by an increase in levels of ADP and/or AMP, which would express a shortage of cell energy (see Adenylate Charge). In addition, most eukaryotic PFKs are strongly inhibited allosterically by ATP, and show nonhyperbolic ATP saturation. Because of this inhibition by ATP, PFK in the liver is almost inactive with the in vivo concentrations of Fru-6P and ATP, and the total PFK activity is much too low to account for the glycolytic flux measured in vivo. This discrepancy between the expected and measured glycolytic flux led to the discovery of the most potent allosteric activator of eukaryotic PFKs, Fru-2,6P2, which relieves allosteric inhibition by ATP (12). The concentration of Fru-2,6P2 in mammalian cells is controlled by hormones that act on cell metabolism through cAMP and protein kinase A. Fru-2,6P2 decreases when cAMP increases. This hormonal control of PFK activity via Fru-2,6P2 regulates the balance between the degradation of glycogen and the synthesis of glucose and/or lipids. Fru-1,6P2 also activates PFKs, but with a much lower affinity than Fru-2,6P2 (12).
Table 1. The Main Effects of PFK in Different Organisms
|
PFKs from |
Activation |
Inhibition |
|
Bacteria |
ADP or GDP3 |
PEP |
|
Mammals- |
AMP.ADP |
Citrate |
|
Fru-2.6P2 |
ATP |
|
|
Yeast |
AMP.ADP |
Citrate |
|
Fru-2.6P2 |
ATP |
a ADP is probably the physiological effector, but GDP has been used to activate bacterial PFKs in many kinetic studies because ADP is also a competitive inhibitor of the substrate ATP.
b The three different isotypes of PFK in mammalian cells are sensitive to the same effectors but to varying extents.
Most eukaryotic PFKs are also allosterically inhibited by citrate, the first metabolite in the Krebs cycle. Citrate inhibition is responsible for the "Pasteur effect" in yeast, a decrease of glucose consumption upon shift from anaerobic to aerobic growth. Indeed, the reoxidation of NADH by oxygen via the respiratory chain raises the level of the Krebs cycle intermediates, and the citrate inhibition of PFK decreases the rate of glycolysis.
Bacterial PFKs are insensitive to citrate, but are sensitive to feedback inhibition by PEP, the penultimate end product of glycolysis.
7.2. Appearance of "New" Regulatory Functions upon Evolution of Sites
The variety of allosteric effectors of PFKs can be explained by their evolution. Bacterial PFKs originally possessed an active site that binds both ATP and Fru-6P, plus an effector site that binds ADP or PEP. After the gene duplication and fusion that doubled the polypeptide chain, the active site on the N-terminal half retained its function, whereas that on the C-terminal active site evolved into a regulatory site (Fig. 1). The Fru-6P subsite became the activation site for Fru-2,6P2 and the ATP subsite became an inhibitory site. Similarly, evolution could have modified an inhibitory site for PEP in bacterial PFKs into one for citrate in eukaryotic PFKs. The evolution from bacterial to eukaryotic PFKs has also created the structural elements needed for communication between the active site and the "new" regulatory sites.
7.3. The Cooperativity of E. coli PFK toward Fru-6P and the Concerted Model of Monod, Wyman, and Changeux
The steady-state kinetics of E. coli PFK were the first to be analyzed according to the concerted allosteric model. Quantitative agreement between experimental data and predictions from the model was remarkable (1). According to the model, the protein is in equilibrium between two conformational states, R and T. The active R state has a much higher affinity for Fru-6P and for the activator ADP (or GDP), whereas the inactive T state has a much higher affinity for the inhibitor PEP. In the absence of ligand, the equilibrium between the R and T states of free PFK largely favors T, and the a ratio T0/R0 = 4*106 (1). The simplicity of the concerted model is that a unique transition between the two states R and T is involved in both the cooperativity toward Fru-6P and the influence of allosteric effectors. Some bacterial PFKs show sigmoi’dal saturations by Fru-6P only in the presence of the inhibitor PEP, confirming that the transition into an inactive state induced by PEP and the cooperativity toward Fru-6P are related.
The crystal structure of the complex between a bacterial PFK and an analog of the inhibitor PEP shows that the latter is bound to a regulatory site remote from the active site and that the protein has changed its quaternary conformation. The change induced by PEP closes the Fru-6P binding site (13). This is consistent with the observation that PEP inhibits by decreasing PFK’s apparent affinity for Fru-6P. Some residues that bind PEP are also involved in binding the allosteric activator ADP when PFK is in its active conformation. The opposite effects of binding activators and inhibitors to a single effector site result because it has two mutually exclusive conformations, that agree with the concerted allosteric model. The quaternary structure of the inactive conformation is less stable, which explains the PEP-induced dissociation of the PFK from T. thermoaquaticus .
However, the transition between the two states R and T seen by X-ray crystallography cannot explain entirely the cooperativity of E. coli PFK toward Fru-6P. In the absence of ligand, free PFK is not in the inactive T0 state because (1) its crystal structure is that of the R state (14), and (2) it binds Fru-6P with high affinity and without any cooperativity (15). Presteady-state kinetics show that binding substrates and/or effectors to E. coli PFK is accompanied by conformational changes within the R state, which are slow enough to be rate-limiting for the catalytic cycle. Thus, the cooperativity of E. coli PFK is in part independent of the transition between the crystallographic R and T states and is due to a change in the rate-limiting step from a conformational change at low Fru-6P concentrations to phosphoryl transfer at high Fru-6P (9). Such a kinetic origin of cooperativity explains observations with some mutants that are difficult to fit with the concerted model, such as values of the Hill coefficient larger than the number of Fru-6P binding sites (16) and reversal of the influence of effectors, where PEP becomes an activator (17) and GDP an inhibitor.
7.4. Phosphorylation of Mammalian PFKs by Protein Kinase A
Mammalian PFKs are phosphorylated on specific sites by protein kinase A. This produces changes in kinetic properties and in the tendency to aggregate into octamers, but the physiological role of this phosphorylation is unknown.