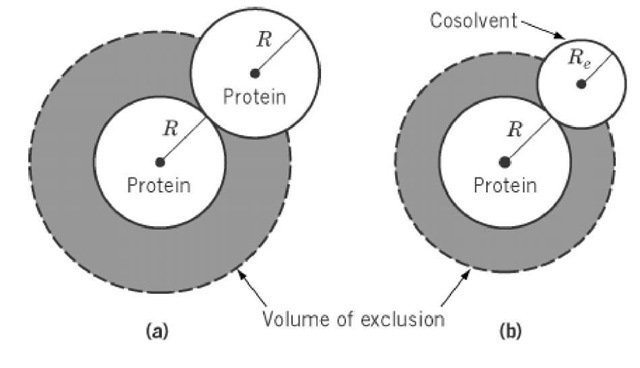
No solute molecule in a solution can be present in the same space as any other molecule in the solvent. This volume of occupation is called the excluded volume V The excluded volume clearly depends on the shape and conformation of the solute molecule. In a dilute solution, the centers of two identical rigid spheres cannot be placed closer to each other than a distance twice as great as their radius (Fig. 1a). Thus, any spherical molecule excludes similar molecules from a volume eight times greater than that it physically occupies. However, the excluded volume due to the interaction of any pair of particles is counted twice, so the volume excluded by all the spherical particles in the system is four times greater than their physical volumes.
Figure 1. Schematic illustration of the excluded volume of spherical molecules. (a) Each solute molecule is excluded from the space occupied by other solute molecules in solution. In very dilute solutions, each molecule of radius R contributes a spherical volume of radius 2 R. (b) A cosolvent of radius Re much greater than that of water statistically cannot be close to a protein molecule due to a steric exclusion principle, resulting in the preferential hydration of protein.
The partial specific (or molar) volume v 2 is usually taken as the physical volume, so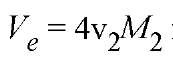 for a spherical solute molecule of molecular weight M2. This should be a good approximation for globular proteins. A rigid rod-shaped solute is regarded as a cylinder, so its Ve is proportional to the length-diameter ratio of the cylinder. The excluded volume of a flexible polymer depends on its radius of gyration, which is sensitive to the polymer-solvent interaction. In a good solvent, solvent molecules are preferably accessible to a polymer, which extends the chain segment and increases the excluded volume. On the contrary, V e is small in a poor solvent, which enhances interactions between different parts of the polymer.
for a spherical solute molecule of molecular weight M2. This should be a good approximation for globular proteins. A rigid rod-shaped solute is regarded as a cylinder, so its Ve is proportional to the length-diameter ratio of the cylinder. The excluded volume of a flexible polymer depends on its radius of gyration, which is sensitive to the polymer-solvent interaction. In a good solvent, solvent molecules are preferably accessible to a polymer, which extends the chain segment and increases the excluded volume. On the contrary, V e is small in a poor solvent, which enhances interactions between different parts of the polymer.
Many polymer solution theories have been developed for the excluded volume of flexible polymers. It is important that the excluded volume be directly related to the second virial coefficient of polymer solutions, so this is an apt criterion for the ideality of the solutions. The excluded volume effect is more significant for nucleic acids at lower salt concentration, where the stiffness or persistence length of the chain segment is influenced by polyelectrolyte effects. The excluded volume effect is also the primary basis for the ability of water-soluble polymers, such as polyethylene glycol, to precipitate proteins. Preferential Hydration is partly due to steric exclusion, in that a larger cosolvent is more effectively excluded from protein surface than water (Fig. 1b).
The excluded volume effect favors any chemical reaction in which the volume decreases. This includes ligand binding by a protein and the association of protein subunits to form oligomers. The equilibrium constant for such a reaction is increased with the increasing concentration of macromolecules in the solution. At high concentrations, such as occur normally in the cytosol of cells, such effects can be very large (2).