The invention: Process that increased the yield of refined gasoline extracted from raw petroleum by using heat to convert complex hydrocarbons into simpler gasoline hydrocarbons, thereby making possible the development of the modern petroleum industry
The people behind the invention:
William M. Burton (1865-1954), an American chemist Robert E. Humphreys (1942- ), an American chemist
Gasoline, Motor Vehicles, and Thermal Cracking
Gasoline is a liquid mixture of hydrocarbons (chemicals made up of only hydrogen and carbon) that is used primarily as a fuel for internal combustion engines. It is produced by petroleum refineries that obtain it by processing petroleum (crude oil), a naturally occurring mixture of thousands of hydrocarbons, the molecules of which can contain from one to sixty carbon atoms.
Gasoline production begins with the “fractional distillation” of crude oil in a fractionation tower, where it is heated to about 400 degrees Celsius at the tower’s base. This heating vaporizes most of the hydrocarbons that are present, and the vapor rises in the tower, cooling as it does so. At various levels of the tower, various portions (fractions) of the vapor containing simple hydrocarbon mixtures become liquid again, are collected, and are piped out as “petroleum fractions.” Gasoline, the petroleum fraction that boils between 30 and 190 degrees Celsius, is mostly a mixture of hydrocarbons that contain five to twelve carbon atoms.
Only about 25 percent of petroleum will become gasoline via fractional distillation. This amount of “straight run” gasoline is not sufficient to meet the world’s needs. Therefore, numerous methods have been developed to produce the needed amounts of gasoline. The first such method, “thermal cracking,” was developed in 1913 by William M. Burton of Standard Oil of Indiana. Burton’s cracking process used heat to convert complex hydrocarbons (whose molecules contain many carbon atoms) into simpler gasoline hydrocarbons (whose molecules contain fewer carbon atoms), thereby increasing the yield of gasoline from petroleum. Later advances in petroleum technology, including both an improved Burton method and other methods, increased the gasoline yield still further.
More Gasoline!
Starting in about 1900, gasoline became important as a fuel for the internal combustion engines of the new vehicles called automobiles. By 1910, half a million automobiles traveled American roads. Soon, the great demand for gasoline—which was destined to grow and grow—required both the discovery of new crude oil fields around the world and improved methods for refining the petroleum mined from these new sources. Efforts were made to increase the yield of gasoline—at that time, about 15 percent—from petroleum. The Burton method was the first such method.
At the time that the cracking process was developed, Burton was the general superintendent of the Whiting refinery, owned by Standard Oil of Indiana. The Burton process was developed in collaboration with Robert E. Humphreys and F. M. This three-person research group began work knowing that heating petroleum fractions that contained hydrocarbons more complex than those present in gasoline—a process called “coking”—produced kerosene, coke (a form of carbon), and a small amount of gasoline. The process needed to be improved substantially, however, before it could be used commercially.
Initially, Burton and his coworkers used the “heavy fuel” fraction of petroleum (the 66 percent of petroleum that boils at a temperature higher than the boiling temperature of kerosene). Soon, they found that it was better to use only the part of the material that contained its smaller hydrocarbons (those containing fewer carbon atoms), all of which were still much larger than those present in gasoline. The cracking procedure attempted first involved passing the starting material through a hot tube. This hot-tube treatment vaporized the material and broke down 20 to 30 percent of the larger hydrocarbons into the hydrocarbons found in gasoline. Various tarry products were also produced, however, that reduced the quality of the gasoline that was obtained in this way.
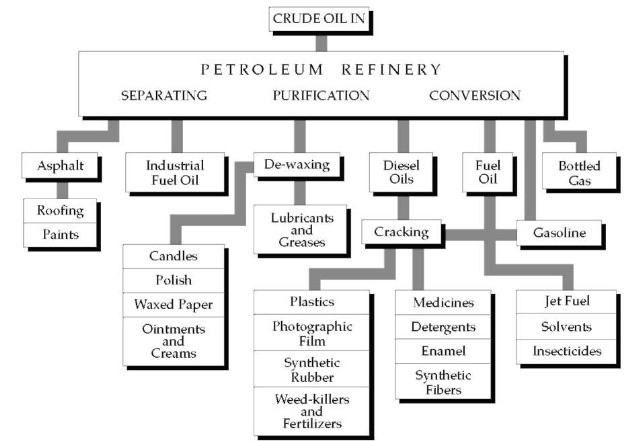
Burton’s process contributed to the development of petroleum refining, shown in this diagram.
Next, the investigators attempted to work at a higher temperature by bubbling the starting material through molten lead. More gasoline was made in this way, but it was so contaminated with gummy material that it could not be used. Continued investigation showed, however, that moderate temperatures (betweenthose used in the hot-tube experiments and that of molten lead) produced the best yield of useful gasoline.
The Burton group then had the idea of using high pressure to “keep starting materials still.” Although the theoretical basis for the use of high pressure was later shown to be incorrect, the new method worked quite well. In 1913, the Burton method was patented and put into use. The first cracked gasoline, called Motor Spirit, was not very popular, because it was yellowish and had a somewhat unpleasant odor. The addition of some minor refining procedures, however, soon made cracked gasoline indistinguishable from straight run gasoline. Standard Oil of Indiana made huge profits from cracked gasoline over the next ten years. Ultimately, thermal cracking subjected the petroleum fractions that were utilized to temperatures between 550 and 750 degrees Celsius, under pressures between 250 and 750 pounds per square inch.
Impact
In addition to using thermal cracking to make gasoline for sale, Standard Oil of Indiana also profited by licensing the process for use by other gasoline producers. Soon, the method was used throughout the oil industry. By 1920, it had been perfected as much as it could be, and the gasoline yield from petroleum had been significantly increased. The disadvantages of thermal cracking include a relatively low yield of gasoline (compared to those of other methods), the waste of hydrocarbons in fractions converted to tar and coke, and the relatively high cost of the process.
A partial solution to these problems was found in “catalytic cracking”—the next logical step from the Burton method—in which petroleum fractions to be cracked are mixed with a catalyst (a substance that causes a chemical reaction to proceed more quickly, without reacting itself). The most common catalysts used in such cracking were minerals called “zeolites.” The wide use of catalytic cracking soon enabled gasoline producers to work at lower temperatures (450 to 550 degrees Celsius) and pressures (10 to 50 pounds per square inch). This use decreased manufacturing costs because catalytic cracking required relatively little energy, produced only small quantities of undesirable side products, and produced high-quality gasoline.
Various other methods of producing gasoline have been devel-oped—among them catalytic reforming, hydrocracking, alkylation, and catalytic isomerization—and now about 60 percent of the petroleum starting material can be turned into gasoline. These methods, and others still to come, are expected to ensure that the world’s needs for gasoline will continue to be satisfied—as long as petroleum remains available.
See also Fuel cell; Gas-electric car; Geothermal power; Internal combustion engine; Oil-well drill bit; Solar thermal engine.
