The mammalian genome contains roughly 3 x 107 CpG dinucleotides, and about 60% of these are methylated at the 5-position of the cytosine. Most 5-methycytosine (m5C) is in transposable elements and their remnants, and removal of methylation by means of mutations in DNA-methyltransferase genes causes the transcriptional activation of transposons in germ and somatic cells. The small fraction of methylation that is not in transposons is involved in the transcriptional repression of certain imprinted genes and in X chromosome inactivation in females. The promoters of tissue-specific genes are not methylated in a pattern that prevents transcription, and the CpG-rich 5′ domains that contain the promoters of 75% of mammalian genes are normally unmethylated at all developmental stages. The common perception of a role for dynamic methylation changes in the regulation of development has not been confirmed, and no gene has been proven to be activated or repressed by reversible DNA methylation. The major biological functions of DNA methylation are transposon repression, monoallelic expression at certain imprinted loci, and X chromosome inactivation in females. Even modest disruption of genomic methylation patterns is lethal to mammals.
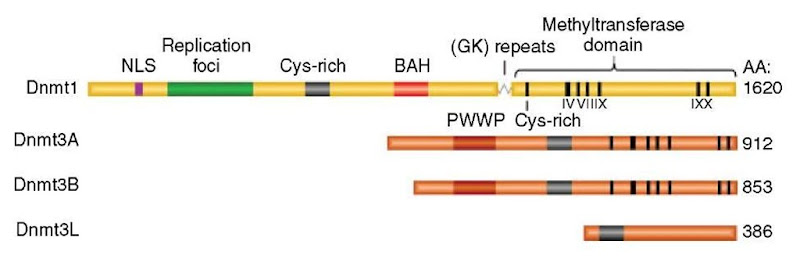
Genomic methylation patterns are established and maintained by DNA (cytosine-5) methyltransferases (DNMTs). As shown in Figure 1, mammals have three enzymatically active DNMTs (DNMT1, DNMT3A, and DNMT3B), a tRNA methyltransferase (RNMT2, formerly DNMT2) that is closely related to DNA methyltransferases in sequence and structure, and DNMT3L, a protein that is related to DNMT3A and DNMT3B in framework sequences but which lacks the catalytic motifs that carry out the transmethylation reaction.
Dnmt3A and Dnmt3B are closely related and have low but approximately equivalent enzymatic activities on unmethylated and hemimethylated substrates (Okano et al., 1998). Deletion of Dnmt3A does not cause detectable alteration of genomic methylation patterns in somatic cells of homozygous mice, although adult mice lack germ cells and die of a condition similar to aganglionic megacolon (Okano et al., 1999). Mice that lack Dnmt3B die as embryos with demethylation of major satellite DNA but normally methylated euchromatic DNA; the Dnmt3A-Dnmt3B double mutant dies very early with demethylation of all genomic sequences in a manner similar to that of Dnmt1 null mutants (Okano et al., 1999). The rare human genetic disorder ICF syndrome (immunodeficiency, centromere instability, and facial anomalies) is due to recessive loss-of-function mutations in the DNMT3B gene (Xu et al., 1999). Patients with ICF syndrome fail to methylate classical satellite (also known as satellite 2 and 3) sequences on the juxtacentromeric regions of chromosomes 1, 9, and 16; these demethylated chromosomes gain and lose long arms at a very high rate to produce the multiradiate pinwheel chromosomes unique to this disorder (Jeanpierre et al., 1993). Dnmt3B is the only mammalian DNA methyltransferase that has been reported to be affected by histone methylation; there is partial demethylation of major satellite DNA (but no reported chromosome destabilization) in mice that lack the heterochromatic histone H3 K9 methyltransferases Suv39h1 and Suv39h2 (Lehnertz et al., 2003). DNA methylation abnormalities have not been reported in mouse embryos that lack the euchromatic histone methyltransferase G9a, nor have there been convincing reports of abnormalities of genomic imprinting, X chromosome inactivation, or transposon silencing in mouse embryos that lack specific histone modifying enzymes (reviewed by Goll and Bestor, 2002, 2005). In mammals, all three processes are dependent on DNA methylation.
Dnmt3A and Dnmt3B are clearly required for the establishment of genomic methylation patterns, but neither enzyme has inherent sequence specificity (reviewed by Goll and Bestor, 2005). The outstanding problem in the mammalian DNA methylation field is undoubtedly the source of the sequence specificity for de novo methylation. Recent studies have shown that unidentified regulatory inputs act through Dnmt3L to guide Dnmt3A and Dnmt3B to target sequences, although the initiating signal or signals remain elusive.
1. Dnmt3L: Expression in male and female germ cells
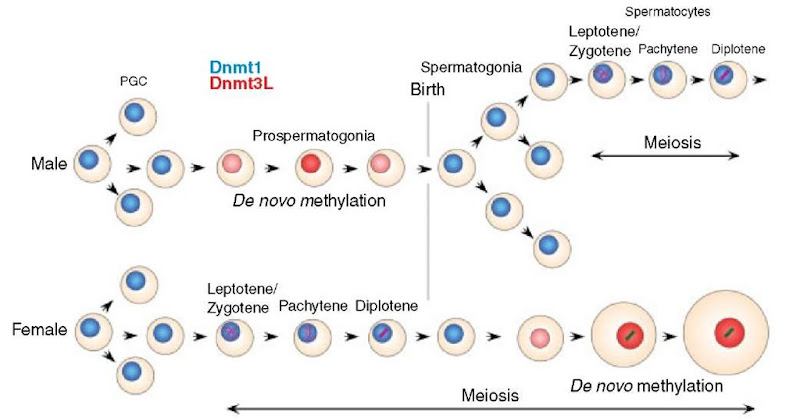
As shown in Figure 2, expression of full-length Dnmt3L is confined to germ cells. The timing of Dnmt3L expression shows striking sexual dimorphism, it is expressed in males only in perinatal prospermatogonia, which will differentiate into spermato-gonia and undergo many mitotic divisions before entering meiosis, but in females expression is limited to growing oocytes, which have completed the pachytene stage of Meiosis I.
1.1. Functions of Dnmt3L in oogenesis
As shown in Figure 1, Dnmt3L lacks the conserved motifs that mediate transmethylation but is related to Dnmt3A and Dnmt3B in framework regions (Aapola et al., 2000). Dnmt3L also fails to methylate DNA in biochemical tests (data not shown). However, Dnmt3L was of special interest because it is the only DNA-methyltransferase homolog whose expression is confined to germ cells at stages at which de novo methylation occurs (Bourc’his et al., 2001).
Figure 1 Structure and motif organization of mammalian DNA cytosine methyltransferases. Dnmt3L is expressed specifically in germ cells and is responsible for guiding Dnmt3A and Dnmt3B to target sequences. See Goll and Bestor (2005) for more information
Figure 2 Expression of Dnmt3L and Dnmt1 in male and female germ cells. Intensity of red coloration indicates levels of Dnmt3L, as evaluated by intensity of f-galactosidase expression in animals heterozygous for a f-geo Dnmt3L knock-in allele (Bourc’his et al., 2001). Dnmt3L is expressed in premeiotic male germ cells but only in postpachytene (midmeiotic) oocytes in females. Dnmt3L is present only at the stages where genomic imprints are established and, in male germ cells, where transposons undergo de novo DNA methylation
Disruption of the Dnmt3L gene by gene targeting in ES cells and insertion of a promoterless f -geo marker into the locus showed that Dnmt3L is expressed in growing oocytes (Bourc’his et al., 2001), the stage at which maternal genomic imprints are established (Kono et al., 1996). We found that mice homozygous for the disrupted Dnmt3L gene were viable and without overt phenotype, although both sexes were sterile. Mutant males were azoospermic, but oogenesis and early development of heterozygous embryos derived from homozygous mutant oocytes was normal; the lethal phenotype was only manifested at e9.5. Such embryos showed signs of nutritional deprivation, and further analysis revealed a failure of chorioallantoic fusion and other dysmorphia of extraembryonic structures (Bourc’his et al., 2001). Analysis of expression of imprinted genes showed a complete loss of imprinting at maternally imprinted loci and a lack of methylation of maternally methylated differentially methylated regions (DMRs). Bisulfite genomic sequencing showed that the imprinting defect was due to a failure to establish genomic imprints in the oocyte, and the normal imprinting of paternally silenced genes in heterozygous offspring of homozygous Dnmt3L-deficient females showed that imprint maintenance in the embryo was normal (Bourc’his et al., 2001). This contrasted with the situation in mice that lack Dnmt1o (an oocyte-specific isoform of Dnmt1), when we found that imprint establishment was normal but imprint maintenance in preimplantation embryos was defective (Howell et al., 2001). Methylation of sequences other than imprinted regions was normal in heterozygous embryos derived from homozygous Dnmt3L mutant oocytes (Bourc’his et al., 2001).
1.2. Functions ofDnmt3L in spermatogenesis
In male mice Dnmt3L is expressed at significant levels only in perinatal prospermatogonia, the stage at which paternal genomic imprints are established (Davis et al., 1999) and transposons undergo de novo methylation (Walsh et al., 1998). Male mice that lack Dnmt3L are outwardly normal except for hypogonadism as adults (Bourc’his et al., 2001). The germ cell population is normal at birth, but only the first cohort of germ cells begins meiosis, and none reach the pachytene stage. All mutant meiotic cells show extreme abnormalities of synapsis; grossly abnormal concentrations of synaptonemal complex proteins and nonhomologous synapsis are obvious in nearly all leptotene and zygotene spermatocytes. Adult males are devoid of all germ cells (Bourc’his and Bestor, 2004). This is in striking contrast to Dnmt3L-deficient females, where meiosis and oogenesis are normal and the phenotype is an imprinting defect apparent in heterozygous offspring of homozygous females (Bourc’his et al., 2001).
The fact that Dnmt3L-deficient male germ cells show a phenotype only after the stage at which Dnmt3L protein is no longer expressed suggests an epigenetic or gene silencing defect. Homozygous mutant male germ cells were purified and found to suffer global demethylation of the euchromatic genome (Bourc’his and Bestor, 2004).
Transposons contain the large majority of m5C present in the mammalian genome (Yoder et al., 1997), and demethylation of the major transposon classes (IAP elements and LINE-1 elements) was observed in Dnmt3L-deficient male germ cells. However, there was little or no demethylation of major or minor satellite DNA when compared with controls. This indicates that the methylation of heterochromatic satellite DNA is controlled by mechanisms distinct from those that control the methylation of euchromatic sequences. Other data support this conclusion; mutations in the DNMT3B gene in humans cause demethylation only of classical satellite (which is analogous to mouse major satellite) in ICF syndrome patients (Jeanpierre et al., 1993; Xu et al., 1999), and the methylation status of major satellite (but not of other sequences) is affected in mice by loss of the histone methyltransferases Suv39h1 and Suv39h2 (Lehnertz et al., 2003), although the magnitude of the effect is much smaller.
The host defense hypothesis predicts that demethylation of transposons in germ cells will cause their transcriptional activation (Bestor, 1990; Yoder et al., 1997; Bestor, 2003). Deprivation of Dnmt3L causes mass reanimation of LINE-1 and IAP transcripts observed by blot hybridization and in situ hybridization (Bourc’his and Bestor, 2004). Dnmt3L is therefore the first gene shown to be required for the silencing of transposons in germ cells of any organism. It is notable that homozygous loss-of-function mutations in Dnmt1 cause reactivation of IAP transcription in somatic cells (Walsh et al., 1998), but LINE-1 elements are not reactivated (Bourc’his and Bestor, 2004); this is likely to reflect the germ cell-specific nature of the promoter in LINE-1 elements (Ostertag et al., 2002). LINE-1 elements are thought to be the source of reverse transcriptase for all retroposons, and the coexpression of IAP elements and LINE-1 elements suggests that active transposition of multiple retroposon classes will occur in Dnmt3L-deficient prospermatogonia and spermatogonia.
Dnmt3L is evolving rapidly by comparison to other mammalian DNA-methyltransferase orthologs (Bestor and Bourc’his, 2004). Rapid evolution often reflects an evolutionary chase in which a parasite evolves at a high rate to escape host defense systems, which are then brought under selective pressures for rapid evolution to counter the innovation of the parasite. Transposons represent the most rapidly diverging sequences within host genomes as a result of incessant selective pressures to evade host defense mechanisms. The rate of evolution of DNA methyltransferases is constrained by the requirement to preserve enzymatic activity. This constraint will limit the diversification of these enzymes and favor the evolution of adapter proteins free of this constraint and therefore capable of evolution at a much greater rate. It is suggested that Dnmt3L arose from an enzymatically active Dnmt3 family member in this way, and that the protein now has a regulatory function and cooperates with other proteins in the recognition and de novo methylation of transposons (in male germ cells) and imprinted genes in both germlines.
2. Conclusion
While recent progress has been significant, a number of outstanding questions remain with respect to the biological function of Dnmt3L and the regulation of de novo methylation in germ cells. Why is Dnmt3L required for the methylation of dispersed repeats and largely dispensable for methylation at DMRs in male germ cells, and why is it dispensable for methylation of dispersed repeats but essential for the methylation of single-copy sequences associated with imprinted genes in female germ cells? What signal does Dnmt3L interpret before recruiting Dnmt3A and Dnmt3B; is it recognition of atypical DNA structures such as cruciforms or homology-heterology boundaries in strand-exchange intermediates? Is it particular patterns of histone modifications, or combinations of proteins of the Polycomb and trithorax groups? Are there pathways that regulate DNA methylation in a manner that is independent of Dnmt3L? The identification of Dnmt3L as a regulator of de novo methylation provides an opportunity to discover interacting factors and to finally identify the cues that designate specific regions of the genome for de novo methylation in germ cells.