Dosage compensation is the process by which the amount of X-linked gene products between individuals with one and two X chromosomes is equalized (see Article 15, Human X chromosome inactivation, Volume 1). Currently, three different mechanisms of dosage compensation are known in nature (reviewed by Marin et al., 2000). In Drosophila, the single X chromosome in males has a twofold increased level of transcription when compared to each of the two X chromosomes in females. In contrast, in Caenorhabditis elegans (C. elegans), a twofold decrease in X-linked gene expression in hermaphrodites (XX) relative to males (XO) ensures equalization of X-linked gene expression.
In mammals, dosage compensation between XY males and XX females is achieved by the transcriptional silencing of one X chromosome in female somatic cells (Lyon, 1961), a process called X-chromosome inactivation (XCI). Therefore, while in Drosophila and C. elegans, the levels of gene expression from each X chromosome in the female or hermaphrodite, respectively, are indistinguishable, a formidable feature of the mechanism of dosage compensation in mammals is that female cells must differentiate the two X chromosomes, rendering only one transcriptionally active.
A snapshot of the inactive X (Xi) in somatic cells would show several epi-genetic modifications of this chromosome when compared to the active X (Xa) (reviewed by Hall and Lawrence, 2003). The Xi is coated by RNA from the Xist gene expressed in cis. It presents higher degree of methylation of CpG islands, higher concentration of the histone variant macroH2A1, and association with the BRCA1 protein. In addition, the histones in the Xi have several posttranslational modifications associated with gene silencing, including hypoacethylation of histones H4, methylation of lysine 9 of histones H3 (H3K9), and dimethylation of lysine 4 of histones H3 (H3K4).
The issue is: how did the Xi get from its originally active state at the zygote to that state of inactivity during early embryonic development? This epigenetic transformation requires that the cell counts the number of X chromosome and chooses which X will be inactive and which will be active so that in a differentiated cell there will be only one Xa per diploid genome.
Counting X chromosomes requires that the cell somehow differentiates the X from the other chromosomes. The identity of the X chromosome is tightly linked to the X-inactivation center (Xic), mapped at Xq13 in humans and in the syntenic region located in band D of the X in mice. Only when this minimal region of the X is present in an X:autosome translocation will the translocated chromosome be counted by the cell as an X to participate in XCI. To date, two genes within the Xic have been demonstrated to be involved in XCI: Xist (X inactive specific transcript), expressed exclusively from the Xi and required for initiation of XCI; and its antisense Tsix, which downregulates Xist in cis in undifferentiated cells.
The choice of which X to be inactive is made in two very different forms in the mouse embryo: in cells of the trophectoderm, the paternal X (Xp) is always chosen to be the Xi, whereas in the inner cell mass (ICM) the choice is random in each cell. Imprinted XCI in the trophectoderm has been associated with maternal imprinting of the Tsix gene. In that lineage, lack of Tsix expression from the Xp leads to stabilization of Xist RNA expressed in cis, triggering inactivation of that chromosome.
In the ICM, although generally either the Xp or Xm is randomly chosen to be inactive, the choice of the Xi may be influenced by the X-controlling element (Xce), located within the Xic. Stronger Xce alleles, which render the corresponding X more likely to remain active, have been described in mice. Mutation studies of the Xist and Tsix genes, also within the Xic, indicate a role for those also in choosing, for instance, a 65-kb deletion 3′ of Xist including most of the Tsix gene leads to primary completely skewed inactivation of the mutant X. In addition, dominant mutations mapped to different mouse autosomes can disrupt normal random XCI. However, the mechanism of choice of the Xi in cells of the ICM is still unknown.
Once the future Xi is chosen, inactivation starts. Until recently, XCI was thought to initiate at cells of the trophectoderm, where the Xp is always inactivated. Subsequently, random XCI would take place in cells of the ICM, where in each cell either the maternal X (Xm) or the Xp is inactivated.
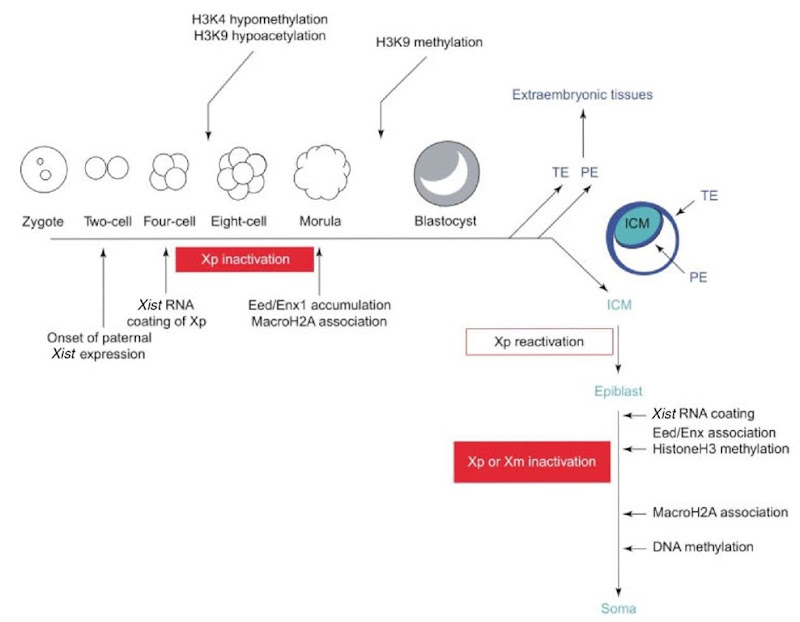
However, more recent studies have detected XCI as early as the cleavage states before cellular differentiation (Okamoto et al., 2004; Mak et al., 2004). At that stage, imprinted XCI takes place in all cells of the embryo, imposing sequential epigenetic modifications in the Xp (Figure 1). These modifications are triggered in cis by the expression of the Xist gene from the Xp. At the 4-cell stage, Xist RNA is first observed, coating the Xp; H3K4 hypomethylation and H3K9 hypoacetylation are detected at the 8-cell stage; at 16-cell stage, the Eed/Enx1 polycomb group complex and the histone variant macroH2A accumulate in the Xp; finally, at the early blastocyst stage, association of methylated H3K9 with the Xp takes place. Together, these epigenetic modifications of the Xp lead to its inactivation.
At the blastocyst stage, while cells of the trophectoderm and the primitive endoderm maintain the inactive state of the Xp, cells of the ICM reactivate this chromosome, erasing the epigenetic marks imposed during the imprinted XCI. Reversible XCI had been reported in embryonic stem (ES) cells carrying an inducible Xist transgene (Wutz and Jaenisch, 2000). In this important experimental model of initiation of XCI, expression of Xist before differentiation leads to Xist -dependent and reversible XCI. Similarly, in the ICM, loss of Xist expression from the Xp leads to dissociation of the Eed/Enx1 complex followed by loss of histone H3K9 and K27 methylation, so that at implantation, the Xp is reactivated.
Figure 1 Kinetics of XCI during early mouse embryonic development. Imprinted inactivation of Xp established before cell differentiation is erased in the ICM. Random inactivation of either the Xp or Xrn will then take place in cells of the epiblast (see text). PE. primitive ectoderm; TE, trophectoderm
At that point, a second round of XCI will take place in cells of the epiblast, starting with repression of Tsix in the future Xi, now chosen at random in each cell, and the consequential stabilization of Xist RNA in cis. The Xist RNA coating the future Xi recruits transiently the Eed/Enx1 complex required for methylation of histones H3, stabilizing the structure of the Xi chromatin. Further modifications of histones H4, recruitment of macroH2A, and methylation of CpG islands will lock that chromosome in an inactive state that is independent of Xist expression and heritable through mitosis.
XCI is traditionally thought of as a mechanism triggered by the presence of supernumerary Xs, involving counting the X chromosomes (or Xics) and randomly choosing the one to be inactivated. Alternatively, XCI can be looked at as a default mechanism in the mammalian cell: X chromosomes will be inactivated, unless they are somehow protected from inactivation. Therefore, the existence of a blocking or protective factor in the cell has been postulated, which must exist in very limited amounts, allowing protection from inactivation of only one X per diploid genome.
The affinity of the protective structure with an X, or with an Xic, may influence the probability of the corresponding X to remain active, that is, it may influence the choice of the Xa. Weaker Xce alleles may have a primary sequence with lower affinity with the protective structure, rendering the corresponding X unprotected from inactivation. Following that rationale, completely skewed inactivation of the X carrying the 65-kb Tsix deletion may be due to total inability of that chromosome to interact with the protective structure. One can also hypothesize that the autosomic factors influencing the choice of the Xi may be part of the protective structure, and therefore, identification of those factors may shed some light on the nature of that structure.
In the last few years, much has been learned about the nature and the dynamics of epigenetic modifications imposed in the Xi during XCI, particularly highlighting the role of posttranslational modifications of histones in defining the epigenetic state of the Xi (see Article 40, Spreading of X-chromosome inactivation, Volume 1). However, the mechanisms by which the female cell chooses and protects one X chromosome from inactivation and the players that impose those epigenetic modifications on the Xi remain obscure and a fascinating topic of research in modern biology.
Finally, it is important to notice that most, if not all, that is known about initiation of XCI comes from studies in the mouse, either in preimplantation embryos or in ES cells. Important differences between XCI in mouse and humans exist, including the apparent absence of a functional human TSIX gene, and of imprinted XCI in human extraembryonic tissues (reviewed by Vasques et al., 2002). Therefore, experimental systems for the study of XCI in humans must be developed. In that sense, the recent availability of human ES cell lines (Cowan et al., 2004) may allow the dissection of initiation of human XCI.