1. Introduction
Twenty years ago, the developmental fate of uniparental embryos was described in mammals. Elegant nuclear transplantation studies in the mouse determined that both a maternal and a paternal genome are required to complete normal development (McGrath and Solter, 1984; Barton et al., 1984). Parthenogenetic and gynogenetic embryos possess only maternally derived chromosomes. The most advanced of these embryos fail to proceed beyond the early limb-bud stage. Extraembryonic tissues are poorly developed. Conversely, androgenetic embryos possess only paternally derived genomes. These embryos rarely advance to the unturned, head-fold stage, and the embryo proper shows poor development. These phenotypes suggest that genes required for embryonic development are expressed from the maternal genome, while genes required for extraembryonic development are transcribed from the paternal genome.
Further analysis indicates that this is a somewhat simplified view. Examination of uniparental embryos and aggregation chimeras revealed phenotypic abnormalities in both embryonic and extraembryonic lineages (Tables 1 and 2). Developmental differences possibly lie in the ability of uniparental cells to proliferate and differentiate. Parthenotes are characterized by a failure to maintain undifferentiated stem cell populations; terminally differentiated cells are overabundant (Sturm et al., 1994; Newman-Smith and Werb, 1995). Androgenetic chimeras display an increased rate of cell proliferation (Fundele et al., 1995). Thus, the original observation of an antipodal effect of androgenetic and parthenogenetic cells on growth and development is supported.
Noncomplementation of parental genomes indicates that transcriptional regulation of specific genes is dependent on germline origin. Two epigenetic phenomena direct monoallelic expression based on parental origin in the mouse, imprinted X chromosome inactivation (XCI), and genomic imprinting.
Table 1 Phenotypic characteristics of uniparental embryos
| Parthenogenetic embryos | Androgenetic embryos |
| Growth retarded Few proliferating cells Overabundant terminally differentiated cells Degeneration of polar trophectoderm Reduced endoreduplication in giant cells Lack ectoplacental cone and extraembryonic ectoderm | Early embryonic growth retardation Trophoblast hyperplasia Hyperproliferation trophoblast giant cells Hyperproliferation chorion Poor infiltration of chorion by allantois Poor infiltration chorionic ectoderm into giant cell layer |
| Thickened extracellular matrix Chorio-allantoic failure Enlarged or bulbous allantois Poor yolk sac vasculature Mesoderm absent/disorganized Neural tube defects Abnormal, small somites Reduced somite number Thinner myocardial layer in heart | Distension of pericardial cavity Weight increase Increased somite number Increase in anterior-posterior length |
Sturm et al. (1994) and Obata et al. (2000).
Table 2 Phenotypic characteristics of uniparental cells in aggregation chimeras
| Parthenogenetic chimeras | Androgenetic chimeras |
| Growth retarded | Weight/size increase |
| Poor yolk sac vasculature | Increase in anterior-posterior length |
| Chorio-allantoic failure | Craniofacial abnormalities |
| Swollen or bulbous allantois | Sternum shortened/compressed |
| Defective labyrinthine development | Vertebrae shortened/thickened |
| Stunted/reduced somite number | Scoliosis |
| Thinner myocardial layer in heart | Rib cartilage hyperplasia |
| Hypo-ossification ribs and skull | |
| Enlarged, distorted, and fused ribs | |
| Shortened limbs | |
| Enlarged and/or fused digits | |
| Postaxial polydactyly | |
| Enlarged and disorganized heart | |
| Umbilical hernia | |
| Abdominal swelling/liver enlargement | |
| Abdominal muscle wall defects | |
| Lack mature brown fat | |
| Eyelid fusion failure |
Spindle et al. (1996), Barton et al. (1991), Fundele et al. (1995) and McLaughlin et al. (1997).
2. Imprinted XCI in extraembryonic development
Dosage compensation of X-linked genes is accomplished by inactivation of one X chromosome in female mammals (see Article 40, Spreading of X-chromosome inactivation, Volume 1 and Article 41, Initiation of X-chromosome inactivation, Volume 1). In rodents, extraembryonic tissues undergo preferential inactivation of the paternal X chromosome (XP). X-inactivation is regulated in cis by the Xist noncoding RNA and its antisense transcript, Tsix (Verona et al., 2003; Takagi, 2003). Xist is transcribed from the paternal X chromosome during preimplantation development and associates with the X chromosome that is to be inactivated, while Tsix is transcribed from the maternal X (XM).
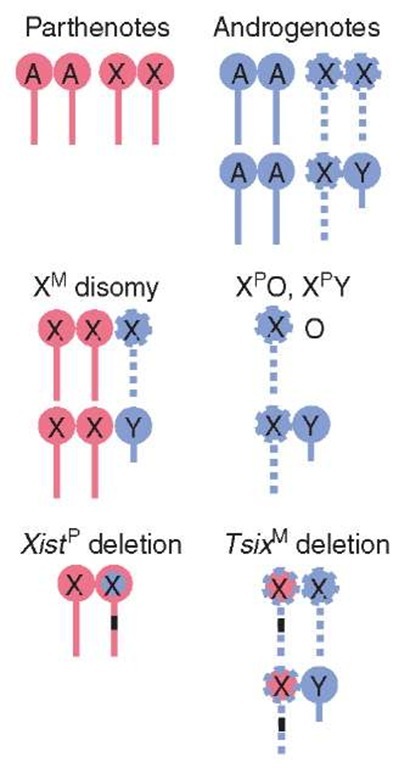
As parthenogenetic embryos possess only maternally derived chromosomes, developmental abnormalities could result from aberrant X-linked gene dosage. In fact, parthenotes fail to undergo XCI; both maternal X chromosomes repress Xist and remain active (Figure 1) (Takagi, 2003). If the phenotype associated with parthenogenesis is due only to the inability to inactivate a second maternal X chromosome, then XMO parthenotes should not suffer the same fate, as X chromosome dosage should be normal. XMO parthenogenetic embryos display the same compromised development as XMXM parthenotes (Mann and Lovell-Badge, 1987), indicating that developmental defects cannot be attributed solely to excessive X-linked gene expression; lack of paternally transcribed, imprinted genes must also contribute to parthenote degeneration.
Conversely, if parthenogenetic failure is due solely to a supernumerary maternal X, embryos with maternal X disomy should be equivalent to parthenotes. XMXMXP and XMXMY embryos repress Xist and possess two active X chromosomes (Figure 1) (Takagi, 2003). Phenotypically, they bear a striking resemblance to parthenotes. Aggregation of maternal X disomic embryos with tetraploid embryos, which contribute functional trophectoderm, rescues this phenotype. Thus, the inability to inactivate an extra maternal X chromosome contributes to early lethality of parthenogenetic embryos by impairing extraembryonic development.
Figure 1 Imprinted XCI in various mouse embryos. Red and blue are maternal (M) and paternal (P) chromosomes, respectively (autosomes: A, X chromosome: X). Solid lines are active and dashed lines are inactive Xs. Black bar indicates deletion
Fatality induced by two active X chromosomes was unequivocally demonstrated by engineering mice with a paternally inherited Xist deletion. Loss of Xist expression results in the paternal X chromosome adopting a maternal epigenotype (Marahrens et al., 1997). Thus, neither the maternal nor the paternal X chromosome is inactivated (Figure 1). Mutant embryos display a remarkably similar phenotype to parthenotes. Mice that are XPO and carry the Xist deletion are normal, demonstrating that it is a second active X chromosome that is responsible for defective trophoblast development.
Similar to parthenotes, aberrant X-linked gene expression may factor into the developmental fate of androgenetic embryos. During preimplantation, androgenotes display ectopic Xist localization from all XP chromosomes, indicative of imprinted XCI (Figure 1) (Takagi, 2003). If the phenotype of androgenetic embryos is due solely to functional nullisomy for the X chromosome, then XPO and XPY embryos should display similar developmental sequelae to androgenotes. These embryos exhibit decreased trophoblast differentiation, developmental delay, and a small ectoplacental cone (Jamieson et al., 1998); their less severe phenotype indicates that lack of expression from maternally transcribed, imprinted genes contributes to androgenetic demise. Reactivation of genes distally located from the X-inactivation center or the X chromosome itself may also ameliorate the phenotype.
One possible explanation for ectopic Xist expression is loss of Tsix expression. Maternal deletion of Tsix leads to derepression of the silent, maternal Xist allele (Lee, 2000; Sado et al., 2001), and the maternal X chromosome takes on a paternal epigenotype (Figure 1). Tsix mutants are characterized by severe embryonic losses and display features common to androgenetic embryos. Early postimplantation, Tsix mutants are developmentally retarded. The ectoplacental cone fails to expand into decidua, and allantois and chorion are disorganized (Sado et al., 2001). One significant difference between androgenotes and Tsix deficient mice is that a small percentage of the latter survive, albeit with growth retardation (Lee, 2000; Sado et al., 2001), indicating that aberrant XCI is not the only determinant in defective extraembryonic development and androgenetic lethality. In summary, extraembry-onic tissues are susceptible to perturbation in X chromosome dosage. However, imprinted XCI alone cannot account for the noncomplementarity of the parental genomes in placental development; genomic imprinting must also play a role.
3. Genomic imprinting during embryogenesis
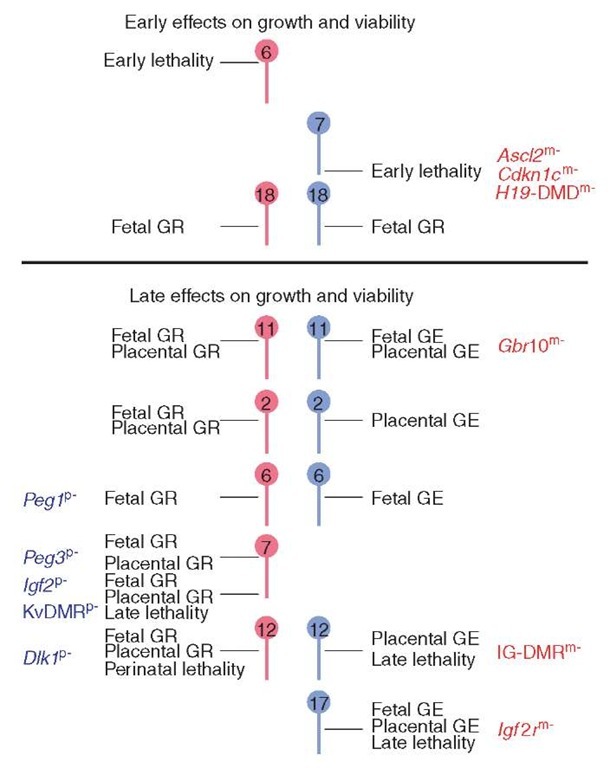
Genomic imprinting is an epigenetic transcriptional regulatory mechanism that is established in the parental gametes and is manifested as parental-restricted expression in developing offspring (Verona et al., 2003). Biological functions for imprinted genes come from studies involving mice with uniparental dis-omy/duplications, and with specific gene deficiencies. Uniparental duplication is a condition in which an individual inherits two copies of a chromosomal region from one parent and no copy from the other parent. An imprinted phenotype may result from overexpression and/or loss of expression of genes that exhibit parental-origin-dependent transcription. Several chromosomal regions affect embryonic development and viability when present from only one parent (Figure 2) (Beechey et al., 2003). Note that fetal and placental growth effects may occur independently and that not every chromosomal region produces a reciprocal effect when the opposite duplication is considered.
Figure 2 Effects of uniparental duplications on fetal and placental development. Only chromosomal region with imprinting effects on embryogenesis are shown (Beechey et al., 2003). Maternal chromosomes/genes are in red, while paternal chromosomes/genes are in blue. GR: growth retardation, GE: growth enhancement. Imprinted genes and imprinting control regions contributing to growth and viability, as determined by targeted mutation, are indicated (m- maternal deletion, p- paternal deletion) (Reproduced by permission of MRC Mammalian Genetics Unit, Harwell, Oxfordshire, Imprinting.
If a specific chromosomal region is responsible for the defective development of uniparental embryos, early lethality should be observed upon uniparental duplication. Only two chromosomal regions are associated with early lethality. Imprinted genes residing on proximal 6 may contribute to parthenogenetic failure (Figure 2). Little is known about maternal duplication for proximal 6 (MatDp(prox6)) except that it is embryonic-lethal prior to day 11.0 of gestation. Genes located on distal 7 may affect the survival of androgenotes as paternal duplication of distal 7 (PatDp(dist7)) is embryonic-lethal around day 9.5 (Beechey et al., 2003). These embryos lack spongiotrophoblast and possess a thickened giant cell layer (McLaughlin et al., 1996). This phenotype is very similar to that of achaete-scute homologue 2 (Ascl2) maternally deficient embryos (Guillemot et al., 1995). Mutant placentas display an increase in giant cells and a deficiency of proliferating spongiotrophoblast that leads to placental failure and lethality.
Three other imprinted genes in the region may also play a role in androgenetic placental development, pleckstrin-homology domain A2 (Phlda2 /Ipl/Tssc3), cyclin-dependent kinase inhibitor 1 C (Cdknlc), and insulin-like growth factor 2 (Igf2). Phlda2 and Cdkn1c are maternally transcribed genes, while Igf2 is expressed from the paternal allele (Verona et al., 2003). Maternal deletion of Phlda2 leads to placental overgrowth due to expansion of spongiotrophoblast and an increase in glycogen cells (Frank et al., 2002). Maternal deletion of Cdkn1c results in an increase in proliferating cells and placentomegaly (Takahashi et al., 2000). Lastly, deletion of the H19 gene including the differentially methylated domain (DMD) results in biallelic expression of the adjacent Igf2 gene that in turn produces somatic and placental overgrowth (Eggenschwiler et al., 1997).
Cdkn1c and Igf2 have been suggested to have opposing effects on cell proliferation (Caspary et al., 1999). Double mutants of Cdkn1c and the H19 DMD display exacerbation of the placental phenotype with overproliferation of trophoblast cells and disruptions in placental architecture, due to excess Igf2 in the absence of Cdkn1c (Caspary et al., 1999). Thus, at least three genes on distal chromosome 7, Acsl2, Cdkn1c, and Igf2, are involved in cell proliferation and differentiation. These genes, as well as Phlda2, may play a pivotal role in aberrant proliferation of androgenetic cells and placental overgrowth. The lethality associated with PatDp(dist 7), however, occurs at a more advanced stage than the majority of androgenetic embryos, indicating the involvement of imprinted genes elsewhere in the genome.
In addition to lethality and placental structure defects, parthenogenetic and andro-genetic cells exert parental-origin effects on growth and development of the embryo proper. Several chromosomal regions are associated with intrauterine growth retardation when maternally duplicated or with intrauterine growth enhancement when paternally duplicated (Figure 2). Targeted deletion studies have identified specific genes that contribute to these growth effects. Loss of maternal-specific expression of growth factor receptor bound protein 10, and of insulin-like growth factor 2 receptor (Igf2r) may account for the fetal and placental growth enhancement of PatDp(prox 11) and PatDp(prox 17) phenotypes, respectively (Beechey et al., 2003; Cattanach et al., 2004). Deficiency for paternally expressed gene 3 (Peg3) and Peg1 may be a factor in MatDp(prox 7) fetal and placental growth retardation and MatDp(subprox 6) fetal growth retardation, respectively (Beechey et al., 2003; Cattanach et al., 2004). In humans, disomy for maternal chromosome 7, which contains the orthologous Peg1 imprinting domain, results in the development of Silver-Russell Syndrome (see Article 26, Imprinting and epigenetic inheritance in human disease, Volume 1), characterized by pre- and postnatal growth retardation, and a small, triangular face (Hitchins et al., 2001).
With respect to specific developmental abnormalities, genes within the gene-trap locus 2 imprinted domain on distal chromosome 12 are likely contributors to skeletal defects and liver enlargement in androgenotes. Paternal disomy for chromosome 12 causes placental growth enhancement, skeletal muscle enhancement, protruding thorax, abdominal extension, enlarged liver, costal cartilage defects, and hypo-ossification (Georgiades et al., 2000). In humans, patients with paternal disomy for chromosome 14 display similar phenotypic characteristics (Kurosawa et al., 2002). Uniparental duplications in mice represent suitable models for investigating human imprinting disorders originating from uniparental disomy (see Article 46, UPD in human and mouse and role in identification of imprinted loci, Volume 1).
Mouse embryos chimeric for PatDp(dist 7) share a large number of developmental anomalies produced by androgenesis (McLaughlin et al., 1997). These same pathogenic features are present in Beckwith-Weidemann Syndrome (BWS), an overgrowth disorder in humans (see Article 26, Imprinting and epigenetic inheritance in human disease, Volume 1 and Article 30, Beckwith-Wiedemann syndrome, Volume 1) involving the orthologous imprinting region (Eggenschwiler et al., 1997). Double mutants for H19 and Igf2r and for H19 and Cdknlc in mice can recapitulate the majority of symptoms seen in BWS patients (Eggenschwiler etal., 1997; Caspary et al., 1999).
Collectively, these data signify that genes within multiple imprinted domains must act synergistically during embryonic development. There is experimental precedence for this. Individually, MatDp(prox 2) and PatDp(prox 11) affect growth but not viability. However, when inherited together, the combination is fatal (Cat-tanach et al., 2004). Additionally, mutations in the maternal H19 DMD on distal 7 and the maternal Igf2r allele on proximal 17 produce an increased frequency and earlier onset of lethality (Eggenschwiler et al., 1997), demonstrating the combinatorial effect of imprinting lesions.
Further evidence for synergism comes from mutations of epigenetic regulators that result in loss of imprinting at multiple loci. DNA methyltransferase 1 (Dnmt1) mutants are growth retarded, and die around embryonic day 9.0 (Lei et al., 1996). Their phenotype is reminiscent of parthenogenetic embryos and may arise from the requirement for Dnmt1 in cell proliferation.
Conversely, Dnmt3a -, Dnmt3a3b-, and Dnmt3L-deficient embryos (see Article 32, DNA methylation in epigenetics, development, and imprinting, Volume 1) display a hyperproliferation phenotype similar to androgenetic fetuses, characterized by pericardial distension, neural tube defects, chorionic-allantoic fusion failure, thickened chorion, and hyperproliferation of secondary trophoblastic giant cells and yolk sac endoderm (Bourc’his etal., 2001; Hata etal., 2002; Kaneda et al., 2004). Defects in these embryos are due to loss of maternal imprints in oocytes. Interestingly, a recessive human disorder, familial biparental complete hydatidiform mole (phenotypically identical to androgenetic moles) occurs from a failure to acquire maternal-specific imprints (El-Maarri et al., 2003).
Bypassing in toto, a double dose of maternal imprinting marks greatly improves development. Gynogenotes generated by nuclear transfer of one nucleus that had and one nucleus that had not been maternally reprogrammed exhibited expression of genes that are normally transcribed from the paternal genome (Kono et al., 2004). However, development was highly variable, a factor likely dependent on the epigenetic state of each immature donor nucleus. Thus, the low rates of viability further substantiate the requirement for a maternal and paternal genome in mammalian development and point to the complex etiology of parthenogenesis and androgenesis.
In conclusion, poor embryonic and extraembryonic development of uniparental embryos is attributed to misregulation of multiple genes governed by genomic imprinting and imprinted XCI. The most important of these genes likely have roles in cell proliferation and differentiation. Parthenogenetic and androgenetic embryos represent good models for investigating genomic imprinting and imprinted XCI. Further studies could provide insight into critical epigenetic events that occur during mammalian embryogenesis, including the stability of imprinted XCI and genomic imprinting in advanced stage uniparental embryos. Furthermore, as andro-genetic embryos possess only paternally derived chromosomes, these embryos may be advantageous for determining the hotly contested question of whether XP is reactivated in the zygote. Finally, given the similarity of developmental defects, investigation of uniparental embryos will be complementary to studies involving interspecific hybrids, somatic cell nuclear transfer embryos (see Article 34, Epige-netics and imprint resetting in cloned animals, Volume 1), and large offspring syndrome (see Article 35, Imprinted QTL in farm animals: a fortuity or a common phenomenon?, Volume 1) that together will further our understanding of epigenetics and mammalian embryogenesis.