The potential applications of gene therapy are plentifold, reaching from hereditary disease to acquired multifactorial disorders. The vector systems used for the transfer of the therapeutic gene into the patient are equally diverse including, for example, naked plasmid DNA as well as engineered viruses or genetically modified cells. Regarded as medicinal product when used in vivo, gene transfer medicinal products (GT-MPs) are unique in their extent of diversity and complexity not only relative to conventional chemical drugs but also relative to biological pharmaceuticals like recombinant proteins. They, therefore, equally challenge research and regulation.
Medicinal products, also termed drugs or medicines, are used for the treatment, diagnosis, or prevention of diseases in or on human subjects. Gene therapy and somatic cell therapy using genetically modified cells are best summarized under the term clinical gene transfer and involve the treatment of human subjects with GT-MPs. GT-MPs belong to the group of advanced biotechnology products for which testing provisions for marketing authorization have been specified in a legally binding document for the European Union. Marketing authorization for a GT-MP was recently granted for the first time worldwide in China for an adenoviral vector transferring the human p53 gene (Pearson et al., 2004). This product is being used for cancer treatment to restore the apoptosis pathway in p53-deficient tumor cells. Data showing clinical efficacy of this GT-MP have not been published.
GT-MPs have been used worldwide by the end of 2004 in almost 1000 clinical trials for in vivo diagnostics, prevention, or therapy. Most clinical gene transfer protocols are aimed at the treatment or prevention of cancer, cardio-vascular disease, infectious disease such as AIDS or monogeneic disorders, or at the prevention of infectious disease by vaccination. The vectors most often used ex vivo are MLV-derived gamma-retroviral vectors, whereas adenoviral and poxviral vectors have mostly been used in vivo. An increasing number of studies involves the use of nonviral vectors or naked DNA (refer to Table in (see Article 95, Artificial self-assembling systems for gene therapy, Volume 2).
More than 6000 human subjects have been treated with GT-MPs worldwide, most of them in the United States. Within Europe, probably the highest number of clinical gene transfer studies have been carried out in the United Kingdom and in Germany. A few clinical trials have resulted so far in benefit for the patients involved (Table 1). It is becoming clear that each disease needs the development of a particular gene transfer method and regiment.
Table 1 Clinical progress in gene therapy
| • | Adenosine deaminase deficiency | Adenosine deaminase gene (ada) | Blood stem cells/retroviral vector | 2 patients cured | Aiuti et al. (2002) |
| SCID-X1 | Gamma-c-chain (IL-2 R) | Blood stem cells//retroviral vector | 10 of 11 babies cured0 | Cavazzana-Calvo et al. (2000), Gaspar et al. (2004) | |
| Peripheral artery occlusive disease | Vascular endothelial growth factor (VEGF) | i.m./plasmid DNA | Improved
vascularization |
Gruchala et al. (2004) | |
| Head and neck tumors | Conditionally replicating adenovirus, no transgene | Tumor cells | Local tumor regression | Post (2002) | |
| Leukemia, graft versus host treatment | Herpes simplex virus thymidine kinase (HSV-tk) | T cells/retroviral vector | Succesful graft versus host treatment | Bonini et al. (1997) | |
| • | Hemophilia B | Factor IX | i.m./AAV vectorb | Improved plasma levels | Couto and Pierce (2003) |
a Two patients developed lymphoproliferative disease due to vector integration. bAAV: adenovirus associated virus.
A particular case was the SCID-X1 trial performed by Alain Fischer’s group at the Hospital Necker in Paris. This clinical trial drew most attention, reaching beyond the gene therapy field. From the same study, spectacular therapeutic effects were reported; however, novel, until then unexpected serious adverse reactions were also reported. SCID-X1 is caused by an inherited disorder, which occurs in the y subunit of cytokine receptors encoded by the y c gene (Fischer et al., 2002). The yc polypeptide is used as a joint subunit in a number of interleukin receptors relevant for hematopoesis, including the receptors for IL-2, IL-4, IL-7, or IL-9. In children with SCID-X1, no functionally active cytokine receptor is found on the surface of lymphocyte precursor cells. This results in a complete failure of the cells to differentiate into T lymphocytes and natural killer (NK) cells. Thus, newborns with this congenital disorder lack the functional immune response to infectious diseases and are therefore forced to live under germ-free conditions. Conventional treatment of this disease requires allogeneic bone marrow transfer, if a suitable donor is available.
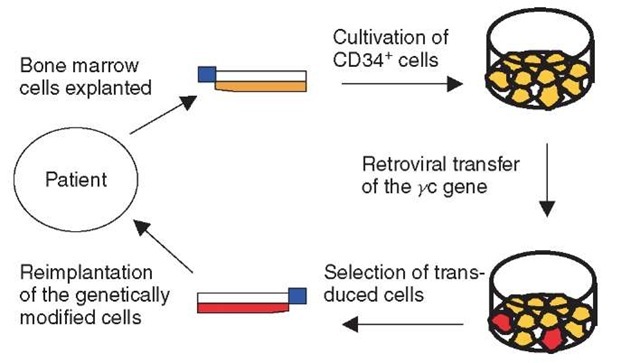
The gene therapy approach uses CD34+ cells isolated from the patient’s bone marrow, activated with a cytokine mixture, and then transduced with the y c gene by means of a conventional replication-incompetent retroviral vector derived from murine leukemia virus (Figure 1). The transduced cells are then reinfused into the patients. Until October 2002, when owing to the observed adverse events the trial was put on hold, 10 SCID-X1 patients had been treated. In nine patients, the gene therapy approach was able to provide a fully functional immune system during an observation period of 3 years, partly even longer. For the affected children and their parents, this meant leading a normal life. The children could even tolerate vaccination against common infectious diseases.
Figure 1 Gene therapy of SCID-X1. The first step is to purify, stimulate, and culture CD34+ cells from the patient’s bone marrow. The cells are then transduced with the yc gene using a retroviral vector, before they are reinfused into the patient
However, about 30 months after treatment, two of the cured patients developed a T cell proliferative syndrome reminiscent of adult T cell leukemia showing a strong increase in the number of T lymphocytes, accompanied by splenomegaly and anemia. Owing to conventional chemotherapy, both patients recovered; one of them is currently in good health but the other patient died because of leukemia (Hacein-Bey-Abina et al., 2003). It is meanwhile commonly accepted that this disease was a result of chromosomal integration of the retroviral vector into the locus of the Imo-2 gene, a known proto-oncogene, resulting in strong overexpression. The Imo-2 gene encodes a transcription factor (rhombotin-2), which is upregulated during hematogenesis but usually not expressed in mature T lymphocytes. Expression of Imo-2, however, can lead to acute T cell leukemia (Herblot et al., 2000). Cofactors besides lmo-2 overexpression that contributed to the T cell proliferation might include the proliferative signals mediated through the transferred yc gene product, as well as familial predisposition to cancer or a chickenpox infection in one of the patients.
Thus, for the first time, insertional oncogenesis had manifested itself in a patient treated with retroviral vectors in the SCID-X1 clinical trial. Cancer development after retroviral gene transfer could therefore no longer be considered exclusively as a theoretical risk. As outlined below, it had immediate consequences for this and also for other clinical gene transfer studies using retroviral vectors.
In the European Union and also in the United States, national authorities are responsible for the registration of clinical trials. In the European Union, Directive 2001/20/EC lays down the rules for good clinical practice (GCP). According to this directive, which had to be transformed into national laws by May 2004, clinical trials using GT-MPs require approval by the competent national authority as well as a positive appraisal by an ethics committee. While the ethics committees, which are usually supported by advice from central gene therapy committees with expert members that cover the different fields of gene therapy, focus on the ethical and medical acceptability of the study, the competent authorities evaluate the acceptability according to the current standards of science. For phase I clinical trials, which is by far the majority of all ongoing clinical gene therapy trials, this includes evaluation of preclinical data on quality, pharmacology, toxicology, and potency of the investigational drug.
The surveillance of clinical trials is another important task carried out by the national authorities. In the event of a suspected risk for enrolled patients or subjects, the competent authority can coordinate and exert suitable measures. Immediately when the reports about the severe adverse reactions in the SCID-X1 trials carried out in France became available, expert hearings took place not only in France but also in other countries such as Germany, the United Kingdom, and the United States to reconsider the safety of retroviral gene transfer. As an initial action (provision), the enrollment of further patients was put on hold at least for all studies using retroviral vectors for blood stem cell modification, in some countries for all clinical gene therapy studies using live retrovirally modified cells. The principal investigators, however, were given the opportunity to review the risk/benefit analysis on the basis of the new results, and to make the appropriate changes in the patient informed consent and the inclusion and exclusion criteria. All studies are currently being continued.
Especially, the risk/benefit analysis is of particular importance in this situation. For life-threatening disease where no alternative treatment is available, even the risk of leukemia may be justified. For example, treatment of patients suffering from chronic granulomatosis, who have a shortened life expectancy due to their congenital immune deficiency disorder, may be continued after changes in the protocol. On the other hand, pure marker gene studies using retroviral vectors without any direct benefit for the patient to be treated currently have a low likelihood of being authorized.