Standard or conventional chromosomal banding analysis is limited to actively dividing cells and the resolution is limited to chromosomal aberrations greater than 3 Mb in size. On the other hand, fluorescence in situ hybridization (FISH) has developed into a meaningful and clinically accepted higher resolution method for analyzing the genetic characteristics of cells. Different FISH technologies provide increased resolution for the elucidation of structural chromosome abnormalities that cannot be resolved by more conventional cytogenetic analyses, including microdeletion syndromes, cryptic or subtle duplications and translocation, complex rearrangements involving many chromosomes, and marker chromosomes. There is a broad array of FISH techniques for both diagnostic and research applications. The FISH procedure has been developed for the tagging of DNA and RNA with labeled nucleic acid probes, and is a process whereby chromosomes or portions of chromosomes are vividly painted with fluorescent molecules that anneal to specific regions. This technique has been used widely for gene mapping and for the identification of chromosomal abnormalities. The method enables enumeration of multiple copies of chromosomes or detection of specific regions of DNA or RNA that represent associations with certain genetic characteristics and infectious diseases. Utilization of the FISH methods requires two essential components. The first is a nucleic acid probe that is complementary to the chromosome region of interest and the second is a buffer that solubilizes the probe and potentiates the denaturation of the cellular proteins and strands of DNA and RNA to be hybridized. The advent of fluorescent dyes for use as labels has made it possible to visualize multiple probes at the same time. This has opened up new possibilities for investigating and diagnosing diseases. There are now whole batteries of commercially available probes that can be used to identify each of the human chromosomes and can be used to identify cells in which chromosomal aberrations occur. Probe hybridization conditions generally depend on the probe’s base-pair sequence and length of bases required to span the region of interest. Generally, probes less than 40 bases long are synthetically manufactured and probes that range from 80 Kb to 1 Mb long are manufactured with molecular biology methods using cosmids, YACs, PACs, or BACs. Probes can also be manufactured with PCR techniques. The FISH probes that are developed in the labs have to be optimized for the FISH procedure. It is also important to compromise between maximizing the sensitivity of the test and minimizing the cost of the study. As hybridization efficiency increases proportionally with the increasing target size, the larger YAC and BAC probes usually give high-efficiency signals, and thus fewer cells need to be scored. In contrast, small single-copy signals hybridize less efficiently; thus, more cells need to be evaluated. The key criteria for development of probes for the FISH technique include bright signal intensity, compactness, and retention of cellular and nuclear morphology. These features provide a signal that is visible at lower magnification, thereby minimizing interference from cross hybridization to other genomic targets. The protocols developed for use with commercially available probes for labeling chromosomes in interphase nuclei and metaphase preparations, are also compatible with synthetically, biologically, or enzymatically lab-produced probes. The buffers used should also be compatible with either direct or indirect labeled fluorescent probes, optimize nucleic acid probe performance for rapid hybridizations to provide bright signals and low background, same-day hybridizations with results ranging from 30 min to overnight depending on probe characteristics.
For all interphase FISH analyses, it is important to establish diagnostic cutoff levels using the probe of choice and a series of normal controls for the type of cell under investigation. These must be established in the laboratory and cannot be adopted from other studies. The addition of different fluorescent labels and the selection of fluorescent dyes have also added variability to the technology method. Further, after labeling with FISH, it is often necessary to scan, detect, and enumerate the fluorescently labeled spots. In order to computerize the images for analysis, it is essential to have low background, specific and uniform labeling, and bright label intensities. After FISH, slides should be stored in the dark, either refrigerated or frozen, and are stable for at least 3 months.
Three different types of probes are commonly used, each with different ranges of applications.
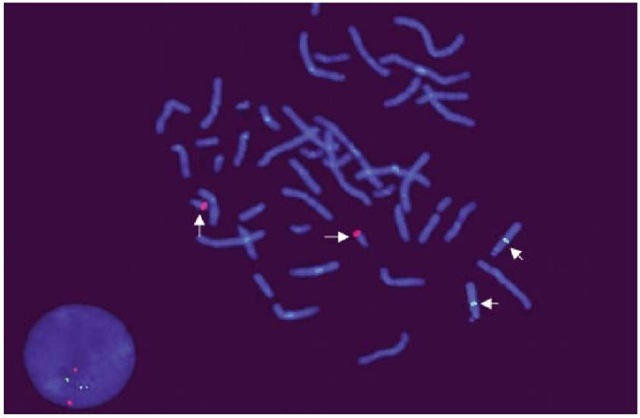
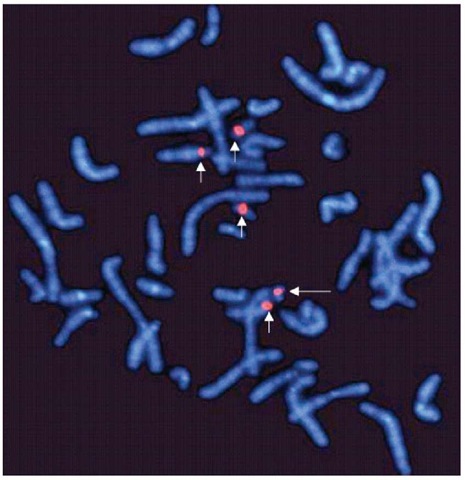
Gene-specific probes target DNA sequences present in only one copy per chromosome. They are used to identify chromosomal translocations, inversions and deletions, contiguous gene syndromes, and chromosomal amplifications in interphase and metaphase chromosomes (Figure 1). Repetitive sequence probes bind to chromosomal regions that are represented by short repetitive base-pair sequences that are present in multiple copies (e.g., centromeric and telomeric probes). Centromeres are usually A-T rich, whereas telomeres are known to have repetitive TTAGGG sequences. Centromeric probes are extremely useful for identifying marker chromosomes and for detecting copy number chromosome abnormalities in interphase nuclei (Figure 2). On the other hand, telomeric probes are frequently used to identify subtle or submicroscopic chromosomal rearrangements. The relative ease of performance and high resolution (0.5 Mb) of repetitive sequence FISH has made it popular to screen for the common chromosomal aneuploidies, subtelomeric deletions, and marker chromosomes.
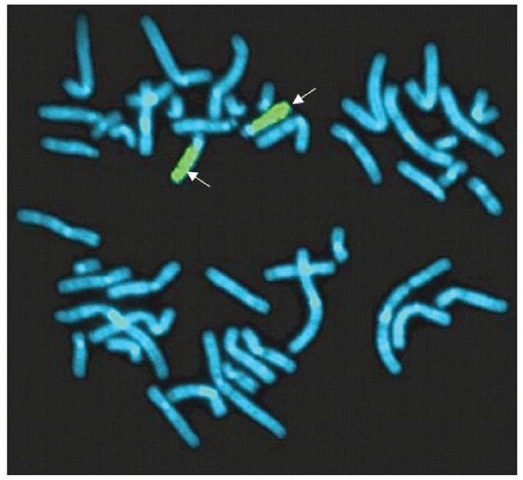
Whole-genome painting probes (WCP) are complex DNA probes that are generated by degenerate oligonucleotide polymerase chain reaction (DOP-PCR) or through flow sorting. WCP paints have high affinity for the whole chromosome along its entire length, with the exception of the centromeric and telomeric regions (Figure 3). These probes are most suitable for identifying genomic imbalances in metaphase chromosomes, especially the complex chromosomal arrangements observed in many cancers. Two variants of the WCP have been developed, multicolor-FISH (M-FISH) and spectral karyotyping (SKY). WCP probes have been developed that paint all human chromosomes in different colors (48 paints). And WCP can also offer simultaneous detection of each arm of all human chromosomes in a single hybridization. This technique is usually used in conjunction with chromosomal banding techniques for a more precise identification of chromosome aberrations. The two greatest limitations of WCP are that there must be extensive knowledge of genetic abnormality to enable the correct selection of the probes -unless the full complement of paints is to be used – and there is limited resolution to detect chromosomal inversions and very small deletions/amplifications and translocations due to its limited resolution of greater than 2-3 Mb.
Figure 1 Locus-specific single-copy DNA probes mapping to the long arm of chromosome 21q22 (Red signals) and chromosome 13q14
Comparative genomic hybridization (CGH) is a molecular cytogenetic technique that emerged from FISH and standard cytogenetics to look for genome-wide DNA copy number changes in patients with a possible chromosomal disorder. This technique is especially valuable because, unlike standard cytogenetics, cell culture is not necessary. CGH can be performed on DNA extracted from archival tissue as well as from fresh-frozen specimens. Chromosomal copy number changes can be detected without having to selectively perform FISH for a specific chromosomal sequence. Briefly, the CGH involves two-color FISH of tumor and reference DNA to normal metaphase chromosomes. In order to prevent nonspecific hybridization, the differentially labeled tumor and control DNA are mixed together with Cot-1 DNA (containing repetitive sequences of the genome). Images of metaphase spreads are obtained with a CCD camera and fluorochrome-specific optical filters to capture the FITC (fluorescein-5-isothiocyanate) and TRITC (tetramethylrhodamine isothiocyanate) fluorescence. The copy number changes in the genome relative to the normal are assessed on the basis of differences in fluorescence intensities along the chromosome. The limitations of this technique include resolution of 3-10 Mb, manual hybridization reactions, and high cost. Recently, array-based formats of CGH (array CGH) have emerged into clinical practice as an alternative. In this procedure, the metaphase chromosomes are replaced with specific DNA sequences spotted in arrays on a glass slide. These DNA sequences may be either large insert genomic clones (e.g., BACs or oligonucleotides-synthesized short DNA sequences. The array readout is digital and quantitative. The greatest advantage of these techniques is the resolution.
Figure 2 Repetitive sequence probes binding specifically to the centromeres of chromosome 14 and 22 (short arrows). The fifth signal (long arrow) shows a marker chromosome derived from either chromosome 14 or 22
The human BAC arrays for CGH consist of linker-adapter PCR representations of BAC clones spotted onto a variety of slide substrates. Hybridization to the human BAC arrays allows detection of single-copy gains and losses. The applications of BAC array technology include identification of diagnostic and prognostic chromosomal aberrations in birth defects, mental retardation, and cancers. The whole-genome analysis usually requires anywhere from several hundred nanograms to a couple of micrograms of genomic DNA, depending on the source of BAC arrays. One commercially available kit for human BAC arrays currently contains 2632 BAC clones distributed approximately uniformly at a spacing of approximately 1 Mb across the genome. Each clone contains at least one sequence tag that is mapped to the human genome sequence. The kit is composed of BACs that are spotted in duplicate allowing greater precision of the results. Clones containing unique sequences near the telomeres and clones containing genes known to be significant in cancer are included. Higher-density whole-genome-and chromosome-specific arrays have been developed in research laboratories with the goal of improving resolution. This slide format uses relatively large amounts of genomic DNA (approximately 6-10 ag at 400-500 ng uL-1) from test specimens for analysis. The signal is highly quantitative and reproducible with low “background noise”. However, the preparation and spotting of thousands of BACs onto slides is cumbersome and labor-intensive. The resolution is limited to the map distance between each clone. The signals produced by low copy, centromeric and telomeric repeats may be nonlinear, and thus nonindicative of the copy number. Array-based CGH technology offers 45 kb to 1 Mb resolution, depending on the density of the array.
Figure 3 A metaphase cell showing two chromosome 15s with whole-chromosome paint probes (arrow, green signal) of chromosome 15
Oligonucleotide-based arrays have gained popularity in the scientific community to monitor the quantitative expression of the arrayed genes in mRNA populations from cell/tissues, and more recently has been applied to detect DNA sequence and copy number polymorphisms in genomic DNA – array CGH. In brief, the arrays contain oligonucleotides matching all wild-type and single nucleotide substitution sequences in a gene, thus providing the basis for detecting single nucleotide polymorphisms (SNPs) as well as genomic copy number polymorphisms. Oligonucleotide array CGH can be applied to testing for mutations, as well as short-and long-range deletions and duplications in the genome, including aneuploidies and gene amplifications. When DNA derived from somatic (i.e., cancerous) cells and normal tissues is compared, loss of heterozygosity events can be detected. The resolution is limited by the density of the oligonucleotide arrays – currently 45-85 kb. Higher-density SNP arrays are currently undergoing ft-testing in several commercial outfits, and these will greatly increase the ability to detect small regions of chromosomal changes and to identify the boundaries of the regions of loss or gain, thereby delineating candidate genes. Additional improvements will come from high-throughput automation of the process, including data analysis, and from development of protocols for handling fragmented DNA.
It is important to emphasize that the FISH methodologies reviewed here complement, but do not replace, standard chromosome banding analysis. Every technique has its strengths and limitations.