1. BWS: historical aspects and clinical features
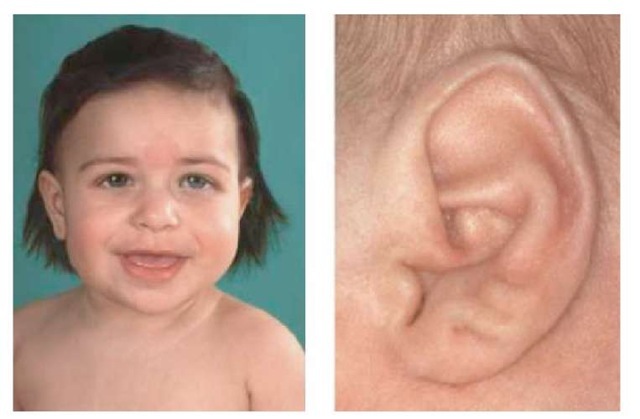
In 1964, Beckwith and, independently, Wiedemann reported a syndrome of macro-glossia and omphalocele, associated with adrenal cortical cytomegaly, fetal gigantism, and other abnormalities. The original abstracts and articles describing these cases have been reviewed recently, along with an interesting discussion of gigan-tism in folklore and early medicine (Beckwith, 1998). The Beckwith-Wiedemann syndrome (BWS, MIM130650), as this constellation of findings came to be named, is thus characterized by overgrowth of many organs during fetal development and, as documented by numerous subsequent reports, an increased susceptibility to childhood tumors. Abdominal wall defects, usually umbilical hernia or omphalocele, can be severe in some cases and require surgical repair after birth. These defects are probably a consequence of the organomegaly, aggravated by a primary defect in the development of the abdominal wall. Macroglossia is often prominent (Figure 1), and partial glossectomy to reduce the size of the tongue is another surgical procedure sometimes performed on children with BWS. In other cases in which glossectomy can be avoided, the jaws grow to accommodate the tongue, making the macroglossia less evident later in life. The kidneys are often increased in size, and they sometimes contain substantial collections of primitive metanephric cells, the so-called nephrogenic rests (Beckwith et al., 1990). No doubt, related to this histological abnormality, BWS is associated with a roughly 7% incidence of Wilms tumor, an embryonal kidney cancer arising from metanephric precursor cells that are defective in their ability to differentiate into mature epithelial structures (Li et al., 2002).
Generalized somatic overgrowth, producing a variable degree of gigantism in the neonate, often accompanied by placentomegaly, is another cardinal feature of BWS. In some cases, the overgrowth can be asymmetrical, producing so-called hemihy-pertrophy, which is probably more accurately termed hemihyperplasia. Lastly, ear pits and creases and facial nevus can be part of the syndrome (Figure 1), and neonatal hypoglycemia is found in about half of BWS cases for which this information is available. As described below, BWS is not a unitary disorder, but instead it has multiple genetic and epigenetic etiologies.
Figure 1 Macroglossia, nevus flameus, and ear creases in a child with BWS
2. Differential diagnosis of BWS: overgrowth syndromes
The clinical differential diagnosis of BWS includes other overgrowth disorders, notably the Simpson-Golabi-Behmel (SGBS), Perlman, and Sotos syndromes, and molecular tests are now a substantive aid in distinguishing these conditions. In Sotos syndrome (MIM117550), caused by deletions and point mutations in the NSDI gene encoding a nuclear regulatory protein, macrocephaly and a characteristic facial gestalt are major consistent features, while overgrowth and advanced bone age are sometimes but not always observed. The Weaver syndrome is a related disorder, and some Weaver syndrome cases have been reported to carry NSDI mutations, making this disorder allelic with Sotos syndrome (Rio etal., 2003). In contrast, SGBS (MIM312870), caused in most cases by mutations in the glypican gene GPC3 (or in other cases the adjacent GPC4 gene) encoding a cell surface proteoglycan, includes somatic overgrowth as a major consistent feature. Renal dysplasia, polydactyly, macrocephaly and coarse facial features, and placentomegaly are additional features of SGBS, which have been comprehensively discussed in the context of a mouse model, the Gpc3 knockout, which largely mimics the human disorder (Chiao et al., 2002). Perlman syndrome (MIM267000), comprising renal hamartomas, nephroblastomatosis, and fetal gigantism, but not omphalocele, is also in the differential diagnosis of overgrowth. Facial dysmorphism and a high perinatal mortality are additional features of this syndrome, which may show autosomal recessive inheritance (Greenberg etal., 1986). Because of its rarity, Perlman syndrome has not yet been defined in molecular terms. In a very complete differential diagnosis, one could also include the Klippel-Trenaunay-Weber syndrome, Proteus syndrome, and even neurofibromatis, because of the hemihyperplasia in this condition, albeit due to vascular malformations. Isolated hemihyperplasia can be a BWS patient with minimal features of BWS. Such individuals have a high tumor risk (5.9%) and should be offered tumor surveillance (Hoyme et al., 1998). Lastly, overgrowth of the fetus is common and well known in the setting of maternal diabetes, but this type of macrosomia does not include omphalocele, macroglossia, or disproportionate visceromegaly; that is, the organ weights, while increased, are appropriate for the overall body size.
3. Genomic imprinting in the BWS region of chromosome 11p15
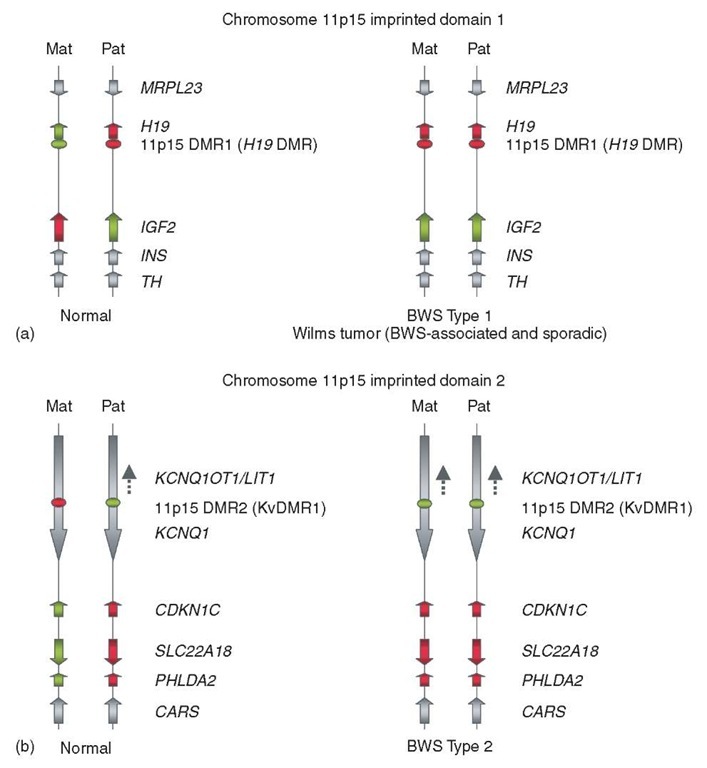
In a broad sense, BWS maps to human chromosome band 11p15, a chromosomal region that contains a large number of imprinted genes. So, to understand the various BWS-associated molecular defects, it is helpful to review the structure and imprinting of this DNA region. The basic concept of genomic or parental imprinting is reviewed elsewhere in this volume (see Article 28, Imprinting and epigenetics in mouse models and embryogenesis: understanding the requirement for both parental genomes, Volume 1 and Article 32, DNA methylation in epigenetics, development, and imprinting, Volume 1); briefly, imprinting is an epigenetic process, occurring in gametogenesis, which marks certain genes for allele-specific, parent-of-origin-dependent, mRNA expression in the conceptus. A large body of evidence implicates DNA methylation at critical CpG-rich sequences as the fundamental epigenetic mark controlling imprinting in mammals. Such “imprinting centers”, which are at most several kilobases in length, control the allele-specific expression of multiple flanking genes, which can be found dispersed in up to several megabases of flanking DNA. Imprinting centers are differentially methylated on the maternal versus paternal chromosome homolog, and are therefore alternatively referred to as “differentially methylated regions” or DMRs (not every differentially methylated DNA sequence is an imprinting center, but the examples discussed here do function in this capacity). As shown in Figure 2, there are two distinct imprinting centers in chromosome band 11p15, one immediately upstream of the H19 gene (the H19 DMR) and the other within an intron of the large KCNQ1 gene (the KvDMR1element, also known as the LITl-associated DMR). The H19 DMR controls the allele-specific expression of H19 and IGF2, while the KvDMRl element controls the allele-specific expression of a larger cluster of imprinted genes, including CDKN1C. Thus, chromosome band 11p15 contains two distinct “imprinted domains”. A list of the imprinted genes in this chromosomal region, with their biochemical functions, is in Table 1, and the transcriptionally active and silent alleles, that is, the direction of imprinting, for several of the relevant genes are diagramed in Figure 2. Most of these genes are maternally expressed/paternally silenced (a pattern often referred to as “paternally imprinted”), but an important exception is the growth-promoting gene IGF2 , which is imprinted in the opposite direction, paternal allele active/maternal allele repressed.
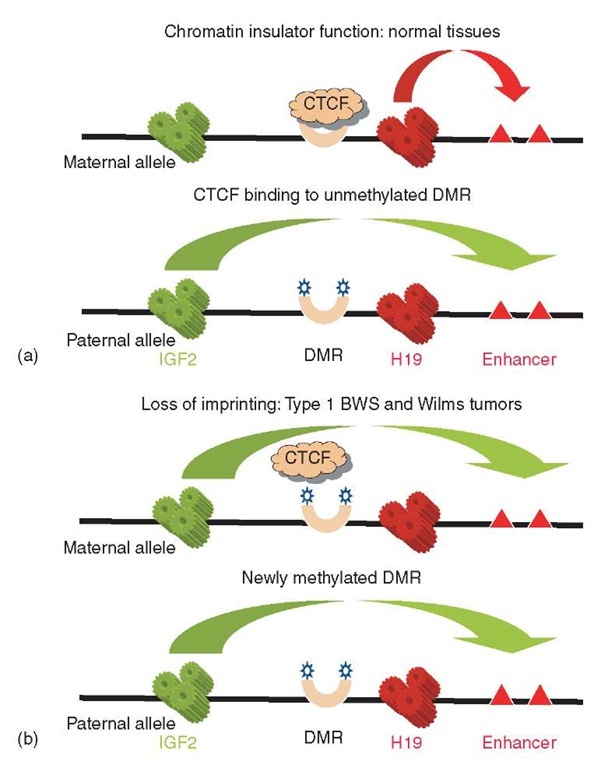
As proven by knockout experiments in mice, each of the two imprinting centers acts in cis to enforce imprinting of its flanking genes (Fitzpatrick et al., 2002; Leighton et al., 1995). Somatic cell genetics using human chromosomes with an engineered deletion of KvDMR1/LIT1 also supports this conclusion (Horike et al.,2000). Several lines of data have led to a credible mechanistic model of how the H19 DMR works to maintain the opposite allele-specific expression of H19 and IGF2 (Thorvaldsen and Bartolomei, 2000). As diagramed in Figure 3, this DMR acts as a chromatin insulator when it is unmethylated (maternal allele), and loses its insulator function when it is methylated (paternal allele). The unmethylated insulator, complexed to an insulator-binding protein called CTCF, blocks the interaction of the IGF2 promoter with downstream enhancer sequences while permitting the H19 promoter to bind to these enhancers. In this situation (maternal allele), H19 nontranslated RNA is produced, while IGF2 mRNA is not. The opposite situation pertains for the paternal allele, in which CTCF binding is prevented by DNA methylation, and the IGF2 promoter is therefore free to interact with the downstream enhancer elements. Precisely how the analogous KvDMRl element acts to maintain imprinting of its flanking genes is not yet clear. Like the H19 DMR, this element is close to the initiation site for a nontranslated RNA (called LIT1/KCNQ1OT1). The function of this RNA is uncertain, but the KvDMR1 element is CG-rich, conserved in mammalian evolution, differentially methylated on the two alleles, and essential for imprinting of distant genes in cis, so it seems likely that some of the principles established for the H19 DMR will also apply to KvDMR1.
Figure 2 Two clusters of imprinted genes in the BWS-associated region of human chromosome 11p15. (a) The more distally located cluster of imprinted genes includes HI9 and IGF2. (b) The more proximally located cluster includes CDKNIC and several other imprinted genes. The red shading indicates lack of expression; green shading indicates active transcription. Gray shading indicates either lack of imprinting, weak tissue-specific imprinting or incompletely characterized imprinting. The cis-acting imprinting control elements (DMRs) are shown as ovals, with green indicating lack of CpG methylation and red indicating substantial CpG methylation. Directions of transcription are shown by the arrows. Mat: maternal chromosome; Pat: paternal chromosome. The abnormalities that lead to Type 1 and Type 2 BWS are indicated
Table 1 Imprinted genes in the BWS-associated region of chromosome 11p15
| Gene | Aliases | Expressed allele | Tissue-specific imprinting | Gene product |
| H19 | Maternal | Many tissues | Nontranslated RNA | |
| IGF2 | Paternal | Many tissues | Trophic growth factor | |
| INS | Paternal | Yolk sac | Insulin | |
| ASCL2 | HASH2 | Maternal (stronger | Extravillus | Transcription factor for |
| imprint in mice) | trophoblast | placental development | ||
| CD81 | TAPA1 | Maternal | Not known | Involved in signal transduction and lymphoma cell growth |
| TRPM5 | MTR1 | Paternal | Not known | Ion-channel |
| KCNQ1 | KvLQT1 | Maternal | Many tissues but not the heart | Ion-channel |
| KCNQ1OT1 | LIT1 | Paternal | Many tissues | Nontranslated RNA |
| CDKN1C | p57KIP2 | Maternal | Many tissues | Cyclin-dependent kinase (cdk) inhibitor; regulates cell cycle and tissue growth |
| SLC22A18 | IMPT1 , ITM | Maternal | Many tissues | Membrane protein, putative multi drug resistance pump |
| PHLDA2 | TSSC3, IPL, | Maternal | Placenta and fetal | PH-domain protein regulating |
| BWR1C | liver | placental growth | ||
| OSBPL5 | ORP5 | Maternal | Placenta | Regulation of sterol metabolism |
| ZNF215 | Maternal | Liver, lung, kidney, testis (not brain and heart) | Zinc-finger protein putative transcription factor |
4. Genetic and epigenetic etiologies of BWS
The phenotypic manifestations of BWS are variable, and this clinical heterogeneity can now be largely explained by an underlying molecular heterogeneity. As shown in Table 2, the majority of BWS cases can be assigned to one of several molecular categories. The observation that some individuals with BWS show mosaic uniparental paternal disomy of chromosome 11 on RFLP (restriction fragment length polymorphism) analysis of blood or fibroblast DNA was an early indication of a role for imprinted genes in this disorder (Henry et al., 1991; Henry et al., 1993), and a substantial minority of cases, about 20%, can be attributed to this abnormality. Similarly, an important early finding was the existence of rare families in which BWS was transmitted by mothers but not fathers, and in which linkage could be established to markers on chromosome 11p15 (Koufos et al., 1989; Ping etal., 1989). In retrospect, most such cases are likely due to either mutations in CDKN1C (Hatada et al., 1996; Lam et al., 1999; O’Keefe et al., 1997) or recently described DNA microdeletions in the CDKN1C enhancer (Niemitz et al., 2004) or the H19 DMR (Sparago et al., 2004). Another small subset of cases is accounted for by rare constitutional chromosomal translocations involving band 11p15, which also cause the syndrome only when transmitted maternally (Mannens et al., 1996). Some of these translocations have DNA breakage and rejoining close to KvDMR1, and presumably silence the expression of CDKN1C (11p15.5) (Lee et al., 1997b), while others influence the expression of a less well understood paternally imprinted gene, ZNF215, in chromosome band 11p15.4 (Alders et al., 2000). Currently, two patients define this more proximal BWS-associated chromosomal region; both demonstrated hemihyperplasia and minimal signs of BWS, and one developed a Wilms tumor.
Figure 3 The chromatin insulator model for opposite imprinting of H19 and IGF2. (a) The insulator binding protein CTCF occupies the HI9 upstream DMR on the maternal allele, thereby preventing the IGF2 promoter from interacting with the shared downstream enhancer elements, and enforcing monoallelic expression of H19 RNA and IGF2 mRNA from opposite alleles. (b) After de novo methylation of the DMR/insulator, IGF2 becomes actively expressed from both alleles, and H19 RNA is lost
Table 2 Genetic and epigenetic defects causing BWS
| Molecular defect | Dysregulated
gene“ |
Proportion of
cases |
Clinical correlations |
| CDKN1C mutation KvDMR1mat loss of methylation | CDKN1C CDKN1C | 3-5% 50% | Omphalocele Omphalocele
Rare tumors other than Wilms |
| H19mat gain of methylation Mosaic patUPD 11p15 | IGF2b
CDKN1C and IGF2 |
10% 20% | Wilms tumors Wilms tumors Hemihyperplasia |
| Chromosomal translocations; chromosome band 11p15 | CDKN1C
ZNF215 dysregulated |
~3% | Not known |
| Trisomy involving chromosome band 11p15 | IGF2 | 5% | Overgrowth Wilms tumors |
| Unexplained (low level mosaicism?) | CDKN1C? IGF2? | 10-15% | Not known |
” See the text for a discussion of additional chromosome 11p15 genes that may contribute to phenotypic variability in BWS.
bH19 noncoding RNA also silenced in these cases.
In distinction to these genetic causes of BWS, two additional large groups of BWS cases are accounted for purely by epigenetic defects (Figures 2 and 3). In about 10% of cases, there is a pathological finding of gain of methylation (GOM) on the maternal H19 DMR. This epigenetic lesion causes loss of imprinting (LOI) of 1GF2, producing a double gene dosage of insulin-like growth factor II (Figures 2a and 3). “Nonsyndromic 1GF2 overgrowth disorder”, also due to tissue mosaicism for GOM at the H19 DMR, but with clinical hallmarks of gigantism and predisposition to Wilms tumor, but not macroglossia or abdominal wall defects, is a forme fruste of this category of BWS (Ogawa et al., 1993; Reeve, 1996). We refer this category of BWS as “Type 1″ in Figure 2. The second epigenetic class of BWS, labeled “Type 2″ in Figure 2, is attributed to loss of DNA methylation (LOM) on the maternal allele of KvDMR1/LIT1 (Lee et al., 1999; Smilinich et al., 1999). As shown in Figure 2, this epigenetic defect leads to pathological biallelic expression of the LIT1/KCNQ1OT1 untranslated RNA, and correlates with transcriptional downregulation (via gain-of-imprinting) of CDKN1C (Diaz-Meyer et al., 2003). Lastly, in about 10-15% of BWS no genetic or epigenetic defect is known. One possibility is that variable tissue mosaicism for LOM at KvDMR1 or GOM at the H19 DMR may underlie some of these cases. Alternatively, some or all of these cases may actually be affected by Sotos syndrome, SGBS, or other BWS mimics.
5. Imprinted genes and growth regulation
From the data discussed above, and from mouse models (Caspary et al., 1999; Eggenschwiler et al., 1997), it is clear that the primary genes in the pathogenesis of BWS are CDKN1C and IGF2. These are good examples of imprinted genes that act to control growth – IGF2 by encoding an antiapoptotic trophic factor with a positive net effect on tissue growth and CDKN1C by encoding a cyclin/cdk inhibitor with a negative net effect on cell proliferation and tissue growth. But the role of imprinted genes in mammalian growth regulation goes well beyond these examples. When this area was reviewed in 2002, there were nine examples of imprinted protein-coding genes proven by in vivo genetic data to control pre- or postnatal growth in mice and/or humans (Tycko and Morison, 2002), and an update from the recent literature reveals at least two additional examples (Charalambous et al., 2003; Moon et al., 2002). Strikingly, for each of these genes, the effect on growth correlates systematically with the direction of imprinting, with paternally expressed genes exerting a positive effect and maternally expressed genes a negative effect on net growth. This correlation is as predicted by the parental conflict theory of imprinting, which was first articulated in the early 1990s after the initial reports of opposite growth phenotypes in mice mutant for the oppositely imprinted Igf2 and Igf2r genes (Moore and Haig, 1991).
Given the large number of imprinted growth-regulating genes in addition to IGF2 and CDKN1C, it is interesting to consider whether aberrant expression of any of these genes might also contribute to overgrowth in humans. On chromosome 11p15 and the syntenic region of distal chromosome 7, in addition to CDKN1C, the PHLDA2 gene (also known as IPL/TSSC3) is a bona fide growth suppressor, with placental overgrowth in Phlda2 knockout mice and placental stunting in conceptuses engineered to overexpress this gene (Frank et al., 2002; Salas et al., 2004). Whether loss of expression of PHLDA2 contributes to placentomegaly in BWS is an open question. While this also needs more study, the insulin gene (INS), closely upstream of IGF2, may well be overexpressed along with IGF2 in the category of BWS with GOM at the H19 DMR. Increased production of insulin may account at least in part for the finding of hypoglycemia in BWS (DeBaun et al., 2000), and since insulin can be mitogenic, such overexpression may also contribute to overgrowth. A related issue is whether any other imprinted chromosomal regions might contain genes that contribute to human overgrowth.
Uniparental maternal disomy for human chromosome 7 produces a growth-retardation syndrome, Silver-Russell syndrome, but we do not yet know of additional recurrent human chromosomal UPDs, other than those involving the chromosome 11p15 BWS region, that lead to overgrowth.
6. Cancer risk in BWS: epigenotype-phenotype correlation
Most cancers encountered in BWS are Wilms tumors (Bliek et al., 2004; Bliek et al., 2001; Gaston et al., 2001), but adrenal cortical carcinomas, hepatoblastomas, rhabdomyosarcomas, neuroblastomas, and other pediatric malignancies are also reported. Perhaps the most striking aspect of heterogeneity in BWS is the fact that cancer occurs only in a small subset, about 7%, of affected individuals. This percentage is lower than that seen in other well-studied human cancer syndromes, such as hereditary retinoblastoma, Li-Fraumeni syndrome, adenomatous polyposis coli and others, an observation that suggests either “incomplete penetrance” or molecular heterogeneity. The latter has proven to be correct, and a welcome recent advance has been the correlation of specific aspects of the BWS phenotype, including cancer risk, with the different molecular etiologies that underlie this disorder.
As early as 1999, a review of the literature suggested that predisposition to Wilms tumor in BWS is high in cases with Chr11p15 UPD or H19 DMR GOM, and low or nonexistent in cases with CDKN1C mutations (Tycko, 1999). This conclusion was confirmed and strengthened shortly thereafter by a number of independent analyses of a large series, including a total of more than 250 molecularly characterized cases, which assessed these three classes of BWS, and also the large fourth category of affecteds with KvDMR1 LOM (Table 3). Frustratingly, the data are analyzed differently in each study, with some investigators choosing to describe the percentages of all tumor-bearing patients that have each molecular abnormality, and others describing the incidence of tumors in patients with a given molecular abnormality. The latter approach is easier to understand, so we have converted the data from each study to this uniform format in Table 3. The reader is also referred to a very recent combined European study of cancer risk in BWS (Bliek et al., 2004). These “meta-analyses” show that Wilms tumors, which in all of the series are the most common cancer, are increased in frequency only in those individuals that have BWS due to Chr11p15 UPD or H19 DMR GOM. Since these two categories of BWS account for a minority of cases, this information nicely accounts for the limited Wilms tumor predisposition.
Larger series of cases will be needed to answer whether the rarer types of BWS-associated neoplasms, including adrenal cortical carcinoma, hepatoblastoma, and neuroblastoma are also specifically associated with a specific epigenotype in BWS. In fact, some of these non-Wilms neoplasms have been identified in BWS cases associated with the KvDMR1 imprinted domain: 2 neuroblastomas have been reported in children with CDKN1C mutations, and 2 hepatoblastomas, 2 rhabdomyosarcomas, and 1 gonadoblastoma were found in BWS-affecteds with KvDMR1 LOM (Lee et al., 1997a; Weksberg et al., 2001).
Table 3 Epigenotype-phenotype correlations in BWS
| Study | Molecular category^ | Tumor6 (n) % | Exomphalosc (n) % | Conclusions |
| Bliek et al. | CDKN1C mutation | Nd | Nd | Neoplasia associated with H19 |
| (2001)d | (n = 1e) | GOM and with UPD11p15, but not with KvDMR1 LOM. | ||
| KvDMR1 LOM | (0) 0% | Nd | ||
| (n = 31) | ||||
| H19 GOM (n = 4) | (2) 50% | Nd | ||
| UPD 11p15 | (3) 27% | Nd | ||
| (n = 11) | ||||
| Undefined (n = 10) | (2) 20% | Nd | ||
| Lam et al. | CDKN1C mutation | (0) 0% | (11) 85% | Exomphalos, but not neoplasia, |
| (1999) | (n = 13/ | with CDKN1C mutation. | ||
| H19 GOM (n = 5) | (0) 0% | (0) 0% | ||
| DeBaun et al. | CDKN1C mutation | Nd | Nd | Neoplasia with H19 GOM and with |
| (2002)g | UPD11p15, but not with KvDMR1 LOM. Exomphalos associated with KvDMR1 LOM. | |||
| KvDMR1 LOM | (1) 3% | (33) 85% | ||
| (n = 39) | ||||
| H19 GOM (n = 10) | (4) 40% | (6) 60% | ||
| UPD 11p15 | (5) 42% | (8) 66% | ||
| (n = 12) | ||||
| Engel et al. | CDKN1C mutation | (0) 0% | (13) 87% | Neoplasia with H19 GOM or |
| (2000) | (n = 15) | UPD11p15, but not with KvDMR1 LOM or CDKN1C mutation; exomphalos only in the latter two categories. | ||
| KvDMR1 LOM | (0) 0% | (20) 69% | ||
| (n = 29) | ||||
| H19 GOM (n = 5) | (1) 20% | (0) 0% | ||
| UPD 11p15 | (2) 9% | (0) 0% | ||
| (n = 22) | ||||
| Gaston et al. | CDKN1C mutation | (1) 50% | (2) 100% | Neoplasia increased in frequency |
| (2001)a | (n = 2) | with H19 GOM, UPD11p15 and trisomy; exomphalos predominantly in cases with KvDMR1 LOM or CDKN1C mutation. | ||
| KvDMR1 LOM | (1) 2.2% | (18) 40% | ||
| (n = 45) | ||||
| H19 GOM (n = 11) | (3) 27.3% | (0) 0% | ||
| UPD 11p15 | (4) 30.8% | (2) 15.4% | ||
| (n = 13) | ||||
| Trisomy 11p15 | 2 | (0) 0% | ||
| (n = 2) | ||||
| Weksberg et al. | CDKN1C mutation | (0) 0.0% | Nd | Wilms tumors in BWS cases with |
| (2001) | (n = 5) | H19 GOM or UPD 11p15; other tumor types (rhabdomyosarcoma, hepatoblastoma, gonadoblastoma) in cases with KvDMR1 LOM. | ||
| KvDMR1 LOM | (5) 14.3% | Nd | ||
| (n = 35) |
Table 3 (continued)
| Study | Molecular categorya | Tumor6 (n) % | Exomphalosc (n) % | Conclusions |
| H19 GOM (n = 3) | (1) 33.3% | Nd | ||
| UPD 11p15 | (6) 28.6% | Nd | ||
| (n = 21) |
aH19 GOM and KvDMR1 LOM refer to cases with these isolated molecular defects; cases with UPD are listed separately.
Incidence of neoplasia within the indicated molecular category of BWS.
cIncidence of exomphalos or, generically, midline abdominal wall defects (exomphalos or umbilical hernia), within the indicated molecular category of BWS.
dThis study reported 7 Wilms tumors and 1 hepatoblastoma in 113 individuals with BWS. Of these, 56 individuals were fully characterized by molecular criteria to assess tumor risk.
eThis single CDKN1C mutant case, found in SSCP screening of 102 patients, had a maternally transmitted missense mutation, but was not further described. This case is not included in the percentages, which are based on 56 fully characterized cases.
f Six patients with Wilms tumor and somatic overgrowth, without classical BWS, were also analyzed. No CDKN1C mutations were found.
gNumbers are extracted from Table 2 of DeBaun et al. (2002) and percentages calculated. Please note that the table in this original publication lists percentages in a different format (% of all BWS-associated tumors accounted for by each category of BWS, rather than tumor incidence in a given category of BWS). Also, in extracting the data from that table, we have segregated the UPD cases, which have both HI9 GOM and KvDMR1 LOM from the non-UPD cases, which have one, but not both, of these molecular defects.
AData are extracted from the text and Table 2 and Figure 3 of this publication. In extracting the data from Figure 3 of that publication, we have segregated the UPD cases, which have both H19 GOM and KvDMR1 LOM from the non-UPD cases, which have one, but not both, of these molecular defects.
7. Developmental defects in BWS: epigenotype-phenotype correlation
The major category of BWS-affecteds with KvDMR1 LOM, or the rarer but seemingly equivalent genetic lesion of CDKN1C mutation, instead of developing Wilms tumors tend to show abdominal wall defects. As is true for the cancer correlations discussed above, the epigenotype-phenotype correlations for abdominal wall defects in all of the large case series analyzed to date are in complete agreement (Table 3).
8. Genetic and epigenetic mosaicism in BWS and Wilms tumor
Paternal UPD for chromosome 11p15 in BWS is found as tissue mosaicism (Slatter etal., 1994), consistent with the known lethal effect of complete paternal UPD for the orthologous chromosomal region in mice (Ferguson-Smith etal., 1991). But tissue mosaicism in BWS is not restricted to this molecular class and is also frequently observed in cases with GOM at the HI9 DMR or LOM at KvDMR1. In these cases, the epigenetic mosaicism is detected as incomplete loss (KvDMR1) or gain (H19 DMR and H19 gene) of specific methylated bands on Southern blots after digesting the DNA with methylation-sensitive restriction enzymes. Whether asymmetrical tissue mosaicism can account for hemihyperplasia/hemihypertrophy in BWS is a difficult question. Mice with mosaic patUPD for the region corresponding to human chromosome 11p15 did not manifest asymmetrical growth and the hemihypertrophy seen in the two translocation cases of BWS with chromosome breakpoints in band 11p15.4 was not accounted for by mosaicism, since the translocations were constitutional (Alders et al., 2000).
As discussed above, tumor predisposition in BWS tracks with gain of DNA methylation at the imprinting center immediately upstream of the HI9 gene, and with paternal uniparental disomy. But this situation is not restricted to BWS: the same types of abnormalities are found as tissue mosaicism in the kidneys of a sizable group of sporadic Wilms tumor patients, who do not manifest the other features of BWS (Moulton etal., 1994; Moulton etal., 1996). In fact, this type of epigenetic mosaicism in the kidneys of children with Wilms tumor is a particularly clear example of a cancer-associated “field effect” determined by early developmental events (Tycko, 2003). What accounts for the de novo GOM in the H19 upstream DMR sequences early in development remains an important unsolved problem.
9. Causal role for epigenetic defects in BWS: discordance in twins
If epigenetic lesions are the primary cause of many cases of BWS, a strong prediction is that monozygotic twins might sometimes be found discordant for this disorder, that is, it should be possible to find rare but informative examples of individuals who share an identical DNA sequence throughout their genomes, but which are nonetheless discordant for the molecular and phenotypic features of BWS. In fact, the older literature contains several case reports of twins discordant for BWS, and a number of these examples were in monozygotic twins (“identical” being a misnomer in this situation) (Hall, 1996; Junien, 1992). The largest single study is a recent compilation of clinical and molecular data for 10 monozygotic twin pairs that were discordant for BWS (Weksberg etal., 2002). As predicted from the hypothesis that BWS is often purely epigenetic in etiology, each of the affected probands showed loss of DNA methylation at KvDMR1. These investigators postulated that there was a failure to maintain methylation of the paternal KvDMR1 allele early in postzygotic development, either coincident or shortly after the twinning event. In addition to this straightforward conclusion, there are two further aspects of BWS and twinning that remain to be explained: there is an excess of females over males among BWS-discordant twin pairs, and the frequency of monozygotic twins with BWS is greater than expected from the rate of twinning in the general population. A discussion of possible explanations for these observations, based on failure of proper subcellular localization and/or function of the DNMT1 methyltransferase enzyme immediately prior to the twinning event, can be found in a recent review (Bestor, 2003).
10. BWS and assisted reproductive technology (ART)
Recent studies from BWS registries in the United States, England, and France have reported a total of 19 cases of this disorder occurring in children produced by in vitro fertilization or intracytoplasmic sperm injection (DeBaun et al., 2003; Gicquel et al., 2003; Maher et al., 2003). On the basis of the total number of cases surveyed, it has been estimated that slightly more than 4% of BWS in developed nations may be associated with assisted reproductive technology (ART). Since it is estimated that ART accounts for only about 1% of all births in these countries, there may be an increased risk of BWS after ART. The denominator in this calculation is uncertain, however, and larger surveys, in which the rate of BWS is studied in unselected pregnancies after ART, are needed. One prior study, concerning 61 pregnancies that were viable to term or late gestation following ART, found a single case of BWS (Olivennes et al., 2001).
While the statistical conclusions will need to be confirmed by larger studies, the available molecular data do provide some support for a causal connection between ART and epigenetic disorders. In particular, 18/19 of the reported ART-associated cases of BWS showed loss of methylation at KvDMR1, with only one case showing GOM at the H19 DMR. This ratio deviates from the expected general distribution of molecular causes of BWS, and the data are suggestive of an ART-related failure of methylation of the maternal KvDMR1 allele, that is, an “oocyte problem” rather than a “sperm problem”. Whether this mechanistic hypothesis can be validated in an experimental system, such as mice conceived by ART, remains to be seen, but further circumstantial support comes from reports of three cases of a second epigenetic disorder, Angelman syndrome, occurring after ART and all showing lack of appropriate DNA methylation of the maternal allele in the relevant chromosome 15 imprinting control region, the SNRPN DMR (Cox et al., 2002; Orstavik et al., 2003).