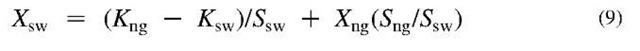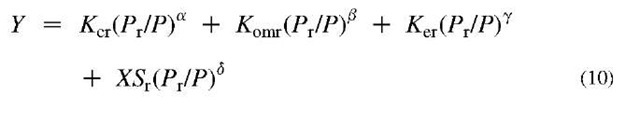Abstract
Solid waste (SW), now mostly wasted biomass, could fuel approximately ten times more of the United States’s increasing energy needs than it currently does. At the same time, it would create good nonexportable jobs and local industries. Twenty-four examples of wasted or underutilized solids that contain appreciable organic matter are listed. Estimates of their sustainable tonnage exceed two billion dry tons. Now usually disposal problems, most of these SWs can be pyrolyzed into substitutes for or supplements to expensive natural gas (NG). The large proportion of carbon-dioxide-neutral plant matter in the list would reduce greenhouse problems. Pyrolysis—heating to high temperatures without oxygen— converts such SW into a medium-heating-value gaseous fuel, usually with small energy expenditure. With advanced gas cleaning technologies the pyrogas can be used in high-efficiency gas turbines or fuel-cell systems. This approach has important environmental and efficiency advantages with respect to direct combustion in boilers and even air-blown or oxygen-blown partial combustion gasifiers. Because pyrolysis is still not a predictive science, the Clean Combustion Technology Laboratory (CCTL) has used an analytical semiempirical model (ASEM) to organize experimental measurements of various product {CaHj,Oe} yields vs temperature (T) for dry ash, nitrogen, and sulfur free (DANSF) feedstock having various weight percentages (wt%) of oxygen [O], and hydrogen [H]. With this ASEM, each product is assigned five parameters (W, T0, D, p, q) in a robust analytical Y{T) expression to represent yields vs temperature of any specific product from any specified feedstock. Patterns in the dependence of these parameters upon [O], [H], a, b, and c suggest that there is some order in pyrolysis yields that might be useful in waste-to-energy conversion (WEC) systems to optimize their throughput. An analytical cost estimation (ACE) model is used to calculate the cost of electricity (COE) vs the cost of fuel (COF) for a SW integrated gasifier combined cycle (IGCC) system for comparison with the COE vs COF for a natural gas combined cycle (NGCC) system. It shows that at high NG prices, SW can be changed from a disposal-cost item to a valuable asset. Comparing COEs when using other SW-capable technologies are also facilitated by the ACE method. Implications of this work for programs that combine conservation with waste-to-energy conversion in efforts to reach Zero Waste are discussed.
SOLID FUELS AND SOLID WASTE
In 1940, when Britain was fighting a ruthless and apparently unstoppable Hitler, Winston Churchill offered only “blood, sweat, toil, and tears” to unite Britain’s political factions. At this time in our history we are excessively (60%) reliant on foreign sources for our liquid fuels and are increasingly importing our gaseous fuels (now > 15%). Our country is shedding blood in its efforts to stabilize regions of the globe that supply these premium fuels. Yet the United States is well endowed with solid fuels in the form of coal and oil shale, and substantial quantities of renewable but wasted solids. In this paper, in continuation of a long search for alternatives to oil,[1-10] our focus is on converting our solid waste to energy by advanced thermal technologies (SWEATT).
Table 1 is a list of the United States’s abundant supply of wasted solids or SW whose organic matter can be made into gaseous and liquid fuels. With recent high natural gas (NG) prices and for technical reasons that will become obvious, this entry will concentrate on advanced thermal technologies (ATT) for the conversions of SW to gaseous fuels. Advanced thermal technologies conversions of coal to liquid and gaseous fuels involve similar technical considerations, but coal to liquid or gas technologies have the attention of many government, business, and engineering personnel. Solid waste to energy by advanced thermal technologies has the attention of only a few in the United States.
In the United States, most of the categories in Table 1 would now be called “biomass” in part because “solid waste” has a bad public image, bringing to mind old incinerators belching black smoke. However, with advances in thermal technologies and gas cleanup systems now being successfully applied in Japan and the European Union (EU),[11] SWEATT deserves a new image. It not only addresses the United States’s very urgent need for alternative fuels, but also could mitigate air and water pollution problems. The large carbon dioxide-neutral plant matter components in Table 1 can help in greenhouse mitigation. The great diversity of physical and chemical characteristics in Table 1 implies that the world needs omnivorous feedstock converters (OFCs) to change these solid fuels into much more usable liquid or gaseous fuels.
Table 1 Wasted solids that could be used as a component of the united state’s primary energy supply
| Waste type | Million dry tons |
| Agricultural residues | ~ 0.98 |
| Forest under-story and forestry residues | ~ 0.40 |
| Hurricane debris | ~ 0.04 |
| Construction and deconstruction debris | ~ 0.02 |
| Refuse derived fuels | ~ 0.10 |
| Urban yard waste | ~ 0.02 |
| Food serving and food processing waste | ~ 0.07 |
| Used newspaper and paper towels | ~ 0.02 |
| Used tires | ~ 0.05 |
| Energy crops on under-utilized lands | ~ 0.05 |
| Ethanol production waste | ~ 0.02 |
| Anaerobic digestion waste | ~ 0.01 |
| Bio-oil production waste | ~ 0.01 |
| Waste plastics | ~ 0.03 |
| Infested trees (beetles, canker, spores) | ~ 0.02 |
| Invasive species (cogon-grass, melaluca) | ~ 0.02 |
| Plastics mined when restoring landfills | ~ 0.03 |
| *Bio-solids (dried pelletized sewage sludge) | ~ 0.04 |
| *Poultry and pig farm waste | ~ 0.02 |
| *Water plant-remediators (algae, hydrilla.) | ~ 0.01 |
| *Muck pumped to shore to remediate lakes | ~ 0.01 |
| Manure from cattle feed lots | ~ 0.01 |
| Plants for phyto-remediation of toxic sites | ~ 0.01 |
| Treated wood past its useful life | ~ 0.01 |
| Total | ~ 2 billion dry tons |
Table 1 is a list of potential local sources of useful nonconventional fuels cited in the author’s conference presentations with recent emphasis on sources available in Florida.Items marked with * help in water remediation and the ~ denotes estimated values.
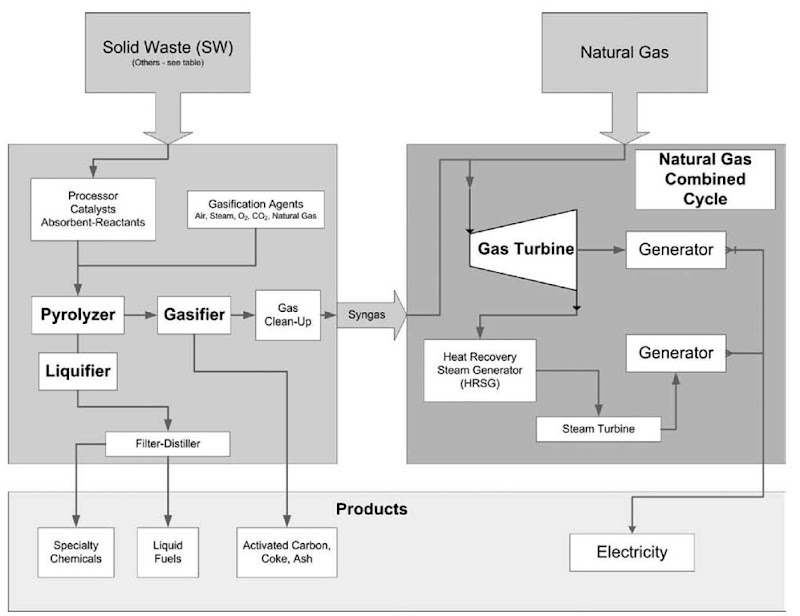
Fig. 1 is a conceptual illustration of an OFC adapted from several previous CCTL papers[8-10]in which a SW pyrolyzer-gasifier-liquifier is co-utilized with a natural gas combined cycle (NGCC) system, as discussed in “ASEM and Pyrolysis.”
PROPERTIES OF SOLID FUELS AND SOLID WASTE
The declining resources of domestic liquid and gaseous fuels are the greatest energy problems facing the United States and many other countries today. Yet, the United States is particularly well endowed with solid fuels in the form of various coals, peat, biomass, and SW. Table 2 shows major ranks of coals as well as of peat, wood and cellulose, and their ultimate and proximate analyses as measured by industry for over a century. The numbers listed in columns 2, 3, and 4 essentially apply to ideal carbon, hydrogen, and oxygen (CHO) materials by correcting measurements to be dry, ash, sulfur, and nitrogen free (DASNF). For such material [C] = 100-[O]-[H] so [C] becomes a variable dependent upon the values of [H] and [O]. Column 5 gives the higher heating value (HHV) in MJ/kg as measured with standard bomb calorimeters after allowing for the minor components.
Fig. 2a is mainly a plot of [H], the wt% of hydrogen (solid diamonds with values read on the left scale) vs [O], the wt% of oxygen, for 185 representative DANSF CHO materials taken from ultimate analysis data available in the technical literature. The bottom scales give conventional coal ranks, some potential names for the biomass region, and some names that might foster more friendly discussions between the coal and biomass communities. This [H] vs [O] coalification plot shows that apart from the anthracite region, all natural DANSF feedstock have [H] values that are close to 6%. The near constancy of [H] together with the relationship [C]= 100- [O]-[H] for DASNF feedstock imply a linear decline of [C] with increasing [O]. The smooth trend of properties with [O] provide strong reasons for regarding peat and biomass simply as lower-rank coals or the various coal ranks simply as aged forms of biomass. The diagram suggests that the natural solid fuels could be ranked simply by [O] to replace the different ranking systems of various countries (a Tower of Babel!). Using 34-O for peat— called “turf’ in Ireland—might help temper the “turf wars” in fuel sector competitions and in energy/environmental confrontations on the use of our available solid fuels.
Higher heating values of various fuels measured with calorimeters usually are reported with proximate analyses. Approximate HHVs in MJ/Kg for the seven representative CHOs are given in Column 5 in Table 2. Column 6 gives representative total volatiles, VT, as determined by an American Standard Test Measurement Method (ASTM). A solid sample is heated (pyrolyzed) in an inert atmosphere using a platinum crucible at 950°C for 7 min. The wt% loss between the sample and its char is the total volatile yield. Then the balance from 100% represents the weight of the fixed carbon (FC) plus ash. When this residual is burned, the remainder is the ash wt%. An empirical analytical formula is given in the caption to represent general trends of total volatiles along nature’s coalification curve. Note the rapidly increasing trend in VT from low [O] materials to high [O] materials. The numbers in Column 7 of Table 2 represent FC = 100 — Vt, the fixed carbon for pure CHO materials after all volatiles are driven off.
Fig. 1 Diagram of the omnivorous feedstock converter (OFC) illustrating the addition of a solid waste (SW) system to an existing natural gas combined cycle (NGCC) plant to create an effective SWCC system.
Columns 8 and 9 of Table 2 give the relative physical density and relative energy density of the various natural solid fuels, which are important factors in handling and transportation costs. Columns 10 and 11 relate to the reactivity and H and OH free radical generated in the combustion of these various solid fuels, which have strong influence on rates of reaction. The last column gives the proposed quantitative [O] ranking system for solid fuels that lie along nature’s coalification curve.
The HHV of a solid fuel is, perhaps, the most important variable in solid fuel use and solid fuel conversion to liquid and gaseous fuels. A simple form of Dulong’s formula, suitable for mental calculations, that captures the main trends is
Divide this number by 2.3 to get the corresponding HHV in MBtu, where M = 1000. Note that the carbon energy term ([C]/3) usually is much larger than the hydrogen energy contribution (1.2[H]). Oxygen contributes negatively in part because the more [O] implies less [C] and in part because of the subtractive term — [0]/10. The larger points on Fig. 2a give the [H], [O] positions of lignin (6.1,32.6), cellulose (6.2,49.4), and hemi-cellulose (6.7,53.3), the three main components of all plant matter. Also shown in Fig. 2a are the [H] and [O] coordinates of several materials that are present in SW. These depart substantially above and below the coalification curve. Not shown are petroleum and polyethylene, which would lie at [14.2, 0],
Fig. 2 (A) Weight percentages of hydrogen [H] vs [O] for 185 DANSF carbonaceous materials (black diamonds) vs oxygen wt%. Classification labels are given at the bottom scale and [O] values on top scale. Adapted from Ref. [4]. (B) Higher heating values (HHV) of 185 carbonaceous materials (corrected to DANSF) vs [O]. The smooth curve represents HHV= ([C]/3+ 1.2[H]-[O]/10). (C) Total volatile weight percentages vs [O] for 185 DASNF carbonaceous materials (squares) from proximate analysis. The curve through the data points satisfies VT = 62([H]/6)([O]/25)1/2. The analytic fixed carbon (FC) is shown.
Table 2 Properties of fuels along natures coalification path
| Ultimate analysis | Proximate analysis | Other properties | H,OH | ||||||||
| Name | C | H | O | HHV | VT | FCCh | Dens | E/vol | RelchR | Rad | O-Rank |
| Anthracite | 94 | 3 | 3 | 36 | 7 | 93 | 1.6 | 58 | 1.5 | v. low | 3-0 |
| Bituminous | 85 | 5 | 10 | 35 | 33 | 67 | 1.4 | 49 | 5 | low | 10-0 |
| Sub bitum | 75 | 5 | 20 | 30 | 51 | 49 | 1.2 | 36 | 16 | med | 20-0 |
| Lignite | 70 | 5 | 25 | 27 | 58 | 42 | 1 | 27 | 50 | interm | 25-0 |
| Peat | 60 | 6 | 34 | 23 | 69 | 31 | 0.8 | 18 | 150 | high | 34-0 |
| Wood | 49 | 7 | 44 | 18 | 81 | 19 | 0.6 | 11 | 500 | v. high | 44-0 |
| Cellulose | 44 | 6 | 50 | 10 | 88 | 12 | 0.4 | 9 | 1600 | v v.high | 50-0 |
Fig. 2b shows the pattern of HHVs vs [O] along the coalification path. The DuLong formula given in the caption is a simple compromise between those used in the coal and biomass sectors. Fig. 2c shows the total volatiles (VT) for the CHO materials vs [O] mostly for materials close to the coalification path. These values are determined by standard proximate analysis procedures that measure the weight loss of a sample after exposure to 950°C for 7 min in an anoxic medium. It should be obvious that in the high [O] region pyrolysis is substantially equivalent to gasification. Detailed studies point to the fact that small departures of [H] from the coalification curve have large impacts on volatile content.
The three diagrams in Fig. 2 all indicate the importance of the [O] in determining the fuel properties of natural substances. CCTL studies indicate that the [H] dimension is also very important in determining volatile content and that small deviations of [H] from the smooth coalification path have a large impact on the volatile release. Table 3 gives a compact list of heating values of various wastes, fuels, and plastics in units of MBtu/lb.
GLOBAL AND U.S. PRIMARY ENERGY SUPPLIES
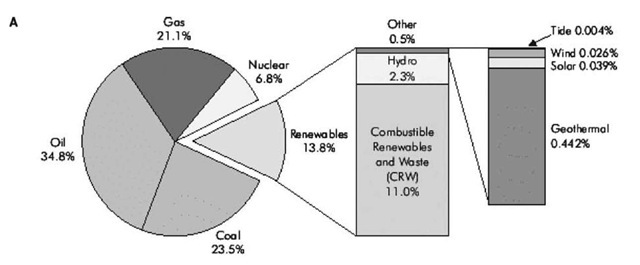
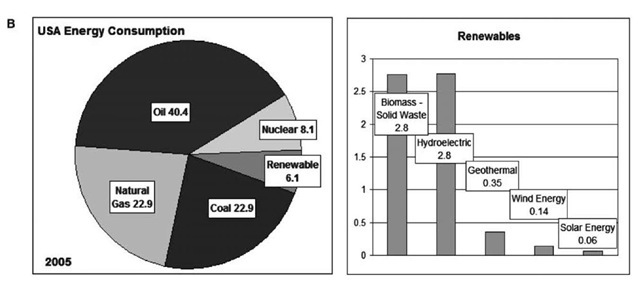
Fig. 3a presents an overview of the world total primary energy supply (see the International Energy Agency Web site) at the opening of the millennia. Among the major sources, combustible renewables and waste (CRW, mostly biomass) need only be doubled to be competitive with coal and NG, and tripled to be competitive with petroleum. Already, the category CRW is almost a factor of 2 greater than nuclear. On the other hand, wind and solar must grow by factors of more than 100 to become major global energy supplies. This global total primary energy supply (TPES) picture is not representative of the industrial world, particularly the United States today. Fig. 3b shows the subdivisions of the U.S. TPES in 2005, in quadrillion Btu or quads (see the U.S. Energy Information Agency Web site).[12] Because total consumption is now very close to 100 quads, the numbers might also be considered to be approximate percentages ofU.S. energy consumption. It is seen that more than 40% of our energy consumption is in the form of petroleum, consumed mainly in our transportation sector. Without doubt the biggest energy problem faced by the United States today is the need to find alternatives to oil.[1-3] In the 1970s and early 1980s, the United States focused heavily on alternatives to oil in the utility sector. The alternatives first were pulverized coal plants and, in the late 1980s and 1990s, NGCC systems. At this time, the United States’ focus should in part be on the developing alternatives to NG for electricity generation via the use of ATT. It should be noted, however, that ATT can also make significant contributions to the solution of our liquid fuel problem in the transportation sector.[3]
Table 3 Heating values of MSW components, fuels, and plastics in 1000 Btu/lb
| Component
Wastes |
As recd | Dry | Component
Fuel |
Dry |
| Paper and paper products | Hydrocarbons | |||
| Paper, mixed | 6.80 | 7.57 | Hydrogen | 60.99 |
| Newsprint | 7.97 | 8.48 | Natural gas | 20.00 |
| Brown paper | 7.26 | 7.71 | Methane | 23.90 |
| Trade magazines | 5.25 | 5.48 | Propane | 21.52 |
| Corrugated boxes | 7.04 | 7.43 | Ethane | 22.28 |
| Plastic-coated paper | 7.34 | 7.70 | Butane | 21.44 |
| Waxed milk cartons | 11.33 | 11.73 | Ethylene | 21.65 |
| Paper food cartons | 7.26 | 7.73 | Acetylene | 21.50 |
| Junk mail | 6.09 | 6.38 | Naphthalene Benzene | 17.30 18.21 |
| Domestic wastes Upholstery | 6.96 | 7.48 | Toluene Xylene | 18.44 18.65 |
| Tires | 13.80 | 13.91 | Naptha | 15.00 |
| Leather | 7.96 | 8.85 | Turpentine | 17.00 |
| Leather shoe | 7.24 | 7.83 | ||
| Shoe, heel, and sole | 10.90 | 11.03 | Oils | |
| Rubber | 11.20 | 11.33 | No. 1 (Kerosene) | 19.94 |
| Mixed plastics | 14.10 | 14.37 | No. 2 (Distillate) | 19.57 |
| Plastic film | — | 13.85 | No. 4 (VL residual) | 18.90 |
| Linoleum | 8.15 | 8.31 | No. 5 (L residual) | 18.65 |
| Rags | 6.90 | 7.65 | No. 6 (residual) | 18.27 |
| Textiles | — | 8.04 | ||
| Oils, paints | 13.40 | 13.40 | Alcohols | |
| Vacuum-cleaner dirt | 6.39 | 6.76 | Methanol | 10.26 |
| Household dirt Food and food waste | 3.67 | 3.79 | Ethanol Plastics | 13.15 |
| Vegetable food waste | 1.79 | 8.27 | ||
| Citrus rinds and seeds | 1.71 | 8.02 | Polyethylene | 19.73 |
| Meat scraps (cooked) | 7.62 | 12.44 | Polystyrene | 16.45 |
| Fried fates | 16.47 | 16.47 | Polyurethane | 11.22 |
| Mixed garbage 1 | 2.37 | 8.48 | Polyvinyl chloride PVC (pure resin) | 9.78 7.20 |
| Coals | Polyvinylidene chloride | 4.32 | ||
| Low-vol. bituminous | — | 15.55 | Polycarbonate | 13.31 |
| Med.-vol. bituminous | — | 15.35 | Cellulose | 7.52 |
| High-vol. bituminous | 12.25 | 14.40 | Polypropylene | 20.22 |
| Subbituminous | 9.90 | 12.60 | Polyester | 12.81 |
| Lignite | 7.30 | 11.45 | ||
| Anthracite | — | 14.00 |
Fig. 3 (A) Total primary energy supply for the globe at the millennium (IEA Web site), (B) (Left) total 2005 annual U.S.A. energy consumption of primary energy sources in quads (Right) renewables.
It is important to differentiate secondary energy supplies (SES) from the primary energy supplies (PES) shown in Fig. 3. Secondary energies supplies include steam, syngas, reactive chemicals, hydrogen, charges in batteries, fuel cells, and other energy sources that draw their energy from PES. If an SES is converted to another type of energy—say, mechanical energy—via a steam turbine, the mechanical energy becomes a tertiary energy supply (TES). This TES can be converted to electrical energy via magnetic generators, in which case the electricity is a quaternary supply (QES). In the case of electricity, the many conversions are usually justified because electricity can readily be distributed by wire and has so many uses as a source of energy for highly efficient electric motors, illumination systems, home appliances, computers, etc.
A debate is under way in many communities as to whether increasing electricity needs should be met with solid fuels—particularly coal—via conventional steam and steam turbine generator systems or via conversion to a gaseous fuel to fuel integrated gasifier combined cycle (IGCC) systems. Granting that the steam turbine route has had many advances over the past century, our thesis is that converting the solid fuel to gaseous fuel is the ATT route of the future. The ATT route is driven not only by environmentally acceptable waste disposal needs and increased needs for electricity, but also by the need for liquid and gaseous fuels. A number of petroleum resource experts recently advanced the date when the globe’s supply of oil and NG will run out. The prices of oil and NG now reflecting this drawdown are already high enough that conversion of organic matter in SW to liquid and gaseous fuels makes economic sense. We should recognize, however, that for the most part, cartels—not free markets—govern fuel prices. Thus, we should not abandon alternative fuels efforts whenever cartels, for their interests, lower prices.
The SWs listed in Table 1, mostly consisting of biomass, now constitute a minor component (~ 2.8%) of the United States’ annual TPES. This wasted material, however, could in the near term become a major (> 25%) component comparable to coal and NG, both now at about 23%. Essentially, the United States now consumes about 100 quadrillion Btu, only about 2.8% of which currently come from SW. The other renewables—hydroelectric (2.8%), geothermal (0.35%), wind (0.14%), and solar (0.06%)—have much further to go than SW before becoming a major primary energy source in the United States. Because SWEATT is based on locally available SW, it would also create good nonexportable local industries and jobs while mitigating serious U.S. energy import and waste disposal problems.
An Oak Ridge National Laboratory study[13] estimates the sustainable supply of the first few biomass categories in Table 1 at about 1.4 billion dry tons. The remaining categories should readily bring the total sustainable U.S. SW available to more than two billion dry tons. Assuming a conservative HHV of 7500 Btu/lb, a simple calculation shows that with SWEATT technologies similar to those that are now in place in Japan, U.S. SW contribution to its primary energy supply could reach the 25% level.
ADVANCED THERMAL TECHNOLOGIES
The largest SW-to-energy systems in operation today are direct combustion municipal solid waste (MSW) incinerators1-14-1 with capacities in the range of 1000-3000 tons per day. In such mass burn systems, the organic constituents of the SW are combusted (in a sense, converted) into the gaseous products CO2 and H2O. These have no fuel value but can be carriers of the heat of combustion, as in coal and biomass boiler-furnace systems. Along with the flame radiation, these gases transfer heat to pressurized water to produce pressurized steam that drives a steam turbine-driven electric generator. The steam also can serve as a valuable secondary energy supply (SES) to distribute heat for heating buildings, industrial processes, etc. The production and use of steam, along with the steam engine, launched the Industrial Age, and various steam-driven systems have reached a very high level of refinement, including in waste-to-energy systems.1-14-1
Solid waste to energy by advanced thermal technologies systems do not involve direct combustion and the use of the heat released to raise steam; rather, the SW is first converted to a gaseous or liquid fuel. Then this fuel serves as an SES that can be combusted in efficient internal combustion engines, combustion turbines, or (in the future) in fuel cells, none of which can directly use solid fuels. Over the past century, automotive and aircraft developments have pushed internal combustion engines (ICE) and gas turbines (GT) to very high levels of efficiency. Furthermore, with the use of modern high-temperature GTs in NG-fired combined cycle (NGCC) systems, the heat of the exhaust gases can be used with a heat recovery steam generator (HRSG) to drive a steam turbine. Alternatively, the HRSG can provide steam for combined heat and power (CHP) system that can effectively make even greater use of the original solid fuel energy.
If one considers the United States’ heavy dependence on foreign sources of liquid and gaseous fuels, the most challenging technical problem facing us today should be recognized as the development and implementation of efficient ways of converting our abundant domestic solid fuels to more useful liquid and gaseous fuels. In view of the diversity of feedstock represented in municipal or institutional SW, any successes in SWEATT would advance this more general quest. In effect, the United States and the world need an OFC such as is illustrated in Fig. 1. Here, the right block represents a typical gas-fired combined cycle system, whereas the left block represents a conceptual omnivorous conversion system that can convert any organic material into a gaseous or liquid fuel.
GROSS COMPARISONS OF ATT OUTPUTS
First, we will consider the gross nature of the output gas from biomass and cellulosic-type material, the major organic components of most solid-waste streams. Apart from minor constituents such as sulfur and nitrogen, the cellulosic feed types are complex combinations of carbon, hydrogen, and oxygen in combinations such as (CgH10Os) that might serve as the representative cellulosic monomer.
Advanced thermal technologies systems may be divided into (1) air-blown partial combustion (ABPC) gasifiers, (2) oxygen-blown partial combustion (OBPC) gasifiers, and (3) pyrolysis (PYRO) systems. The three approaches for converting waste to a gaseous fuel have many technical forms, depending on the detailed arrangements for applying heat to the incoming feed and the source of heat used to change the solid into a gas or liquid.
Let use “producer gas” as a generic name for gases developed by partial combustion of the feedstock with air, as in many traditional ABPC gasifiers that go back to Clayton’s coal gasifier of 1694. We will use “syngas” for gases developed by partial combustion of the feedstock with oxygen, as in OBPC gasifiers, which are mainly a development of the 20th century. We will use “pyrogas” for gases developed by anaerobic heating of the feedstock, such as in indirectly heated (PYRO) gasifiers. Our objective is to replace NG that has HHV~ 1000 Btu/ ft3 = 1 MBtu/ft3 (here, M = 1000).
When an ABPC gasifier is used with cellulosic materials (cardboard, paper, wood chips, bagasse, etc.), the HHV of biomass producer gas is very low (100- 200 Btu/ft3) for two reasons: (1) The main products are CO that has a HHV of 322 Btu/ft3 and CO2 and H20 that have zero heating values and (2) the air nitrogen substantially dilutes the output gas.
The syngas obtained from biomass with an OBPC gasifiers is better—~ 320 Btu/ft3 because it is not diluted by the atmospheric nitrogen. It is still somewhat lower than the feedstock molecules, however, because of the partial combustion. The oxygen separator is a major capital-cost component of an OBPC gasifier.
With a PYRO system, the original cellulosic polymer is first broken into its monomers, leading to some CO, CO2, and H2O along with paraffins (CH4, C2H6, and C3H8…), olefins (C2H4, C3H6,…) and oxygenated hydrocarbons: carbonyls, alcohols, ethers, aldehydes and phenols, and other oxygenated gaseous products. Cellulosic pyrogas can have heating values in the 400 Btu/ft3 range.
Hydrocarbon plastics such as polyethylene and poly-olefins in general are among the most predominant plastics in many SW streams. Thus, one might use (C2H4) as representative of the monomers in the plastic component of MSW or refuse-derived fuels (RDF). Polyethylene pyrolysis products include H2, olefins, paraffins, acetylenes, aromatics (Ar), and polynuclear aromatics (PNA). On a per-unit weight basis, all but H2 have gross heating values in the range 18-23 MBtu/lb (M = 1000), similar to oil, whereas H2 has a gross heating value of 61 MBtu/lb. On a per-unit volume, basis all polyethylene pyrolysis products have gross heating value ranging from 1 to 5 MBtu/ft3, whereas H2 is 0.325 MBtu/ft3 = 325 Btu/ft3. Natural gas typically is about 1 MBtu/ft3. Thus, we would expect the pyrogas from polyethylene to have a gross heating value comparable to or greater than that of NG, and much greater than that of cellusosic pyrogas.
In summary, because cellulosic feedstock is already oxygenated as compared with pure hydrocarbon plastics, its pyrogas, syngas, and producer gas will all have considerably lower heating values than the corresponding gases from hydrocarbon feedstock. From the viewpoint of maximizing the HHV of SW-derived gas, PYRO gasification scores better than OBPC gasification, which scores better than ABPC gasification.
ASEM AND PYROLYSIS
Proximate analyses of coal and biomass measured for more than a century provide extensive data on total volatile content. A predictive method for identifying the molecules in these volatiles is still not available, however, despite the fact that such knowledge could provide a fundamental understanding of humankind’s oldest technology: the use of fire. For control and application of a pyrolysis system, it would be useful to have at least an engineering-type knowledge of the expected yields of the main products from various feedstock subjected to anaerobic thermal treatment.
In most attempts to find the systematic of pyrolysis yields of organic materials such as coal and biomass, including the initial CCTL studies,[15-20] it has been customary to characterized the feedstock by its atomic ratios y = H/C and x = O/C. In its recent studies,[21-27] the CCTL has found it more advantageous to work with the weight percentages [C], [H], and [O] of the feedstock after correcting to dry, ash, sulfur, and nitrogen free (DASNF) conditions (i.e., pure CHO materials). These were attempts to find some underlying order of pyrolysis yields of any product CaHfoOc vs the [O] and [H] of the DASNF feedstock and the temperature (T) and time (t) of exposure. In organizing CCTL pyrolysis data as well as data in the literature, the CCTL has developed an analytical semi-empirical model (ASEM) that has been useful for several applications of pyrolysis.[19-27] Some progress has been made in including the time dimension, but much more work remains. When the time dimension is not a factor, the yields of each product for slow pyrolysis (or fast pyrolysis at a fixed time) are represented by
Here, L(T) is the well-known logistic function that is often called the learning curve. Thus, its complement F(T) = 1 — L(T) might be called the forgetting curve. For engineering applications, this curve-fitting approach provide a more robust and convenient means for organizing pyrolysis data than traditional methods that use conventional Arrhenius reaction rate formulas.[28] In the ASEM, each product is assigned five parameters (W, T0, D0, p, q) to represent its yield-vs-temperature profile. The objective has been to find how these parameters depend on the [H] and [O] of the feedstock and the a, b, c of the CaHbOc product for the data from particular types of pyrolyzers. Studies by Xu and Tomita (XT)[29] that gave data on 15 products from 17 coals at six temperatures have been particularly helpful in revealing trends of the parameters with [O] and [H]. In applying the ASEM to the CCTL data collection, the XT collection, and several other collections, a reasonable working formula was found for the yield of any abc product for any [O], [H] feedstock. It was given by
where z = [C]/69, h = [H]/6, and x = [O]/25, and the parameters a, , and y, T0, D, p, and q were found to have simple relationships to the feedstock and product defining parameters [H], [O], a, b, and c. The final ASEM formulas that fit the data could then be used to extrapolate or interpolate the XT results to any [H], [O] feedstock and temperature. Fig. 4 gives an overview of the interpolated and extrapolated Y{T) outputs for a selection of products for four representative feedstock along nature’s coalification path.
Fig. 4 Wt% yields vs temperature (in °C) from pyrolysis of anthracite, bituminous, lignite, and wood with ([C], [H], [O]) as shown. HC represents C2 and C3 gasses, BTX, phenol and cresol.
Because hundreds or even thousands of organic products of pyrolysis have been identified in the literature, to go much further, some comprehensive organization of these products is needed. Toward this goal, the CCTL has grouped products into the families shown in Table 4, along with the a, b, and c rules that connect these groups. This list can be subdivided into pure hydrocarbons (i.e., CaHb) and the oxygenates (CaHfoO, CaHfo02, CaHfo03, etc). Isomers (groups with identical a, b, and c) can differ in detailed pyrolysis properties and, hence, parameters. We use j = 1, 2, 3, etc. to denote the first, second, third, etc. members of each group or the carbon number (n). In the CCTL’s most recent studies[21-27] of specific feedstock pyrolysis, formulas have been proposed and tested for the dependence of the W, T0, D0, p, and q parameters on the carbon number of the product within each group. This makes it possible to compact a very large body of data with simple formulas and a table of parameters.
Table 4 Organization of functional groups by family
| Families | a | b | c |
| Paraffins | j | 2a + 2 | 0 |
| Olefins | j +1 | 2a | 0 |
| Acetylenes | j +1 | 2a – 2 | 0 |
| Aromatics | 5 + j | 4 + 2j | 0 |
| Polynuclear | 6 + Aj | 6 + 2j | 0 |
| Aldehydes | j +1 | 2a | 1 |
| Carbonyls | j | 2a | 1 |
| Alcohols | j | 2a + 2 | 1 |
| Ethers | j +1 | 2a + 2 | 1 |
| Phenols | 5 + j | 4 + 2j | 1 |
| Formic acids | j | 2a | 2 |
| Guaiacols | 6 + j | 6 + 2j | 2 |
| Syringols 1 | 7+ j | 8 + 2j | 3 |
| Syringols 2 | 8+ j | 10 + 2j | 4 |
| Sugars 1 | 4+ j | 10 | 5 |
| Sugars 2 | 5+ j | 10 + 2j | 5 |
a, b, and c are the subscripts in CaHbOc, where j = 1, 2, 3….
a, b, and c are the subscripts in CaHbOc, where j = 1, 2, 3….
The case of polyethylene is an example of such a study. It is not shown in Fig. 2a, as it is far removed from the coalification curve, having the position [H] = 14.2 on the [O]= 0 axis. Without oxygen in the feedstock, the pyrolysis products are much fewer, and the ASEM is much simpler to use than with carbohydrates. Thus, only the first five rows of Table 4 are needed to cover the main functional groups involved in organizing the pyrolysis products of polyethylene.
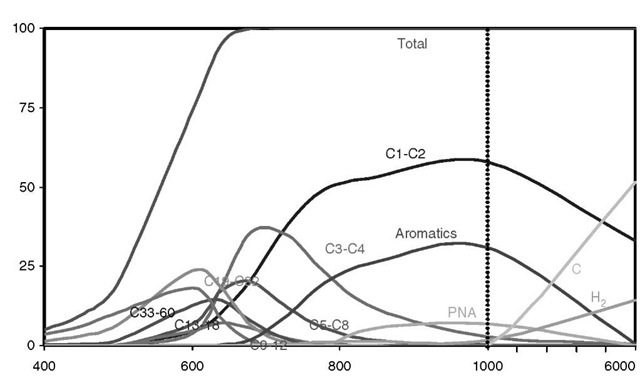
Fig. 5 gives an ASEM-type summary of the product yields vs temperature based on fits to the experimental data of Mastral et al.[30'31] at five temperatures that were constrained to satisfy approximately mass, carbon, and hydrogen balances. When the parameter systematics are identified, the ASEM representation can be used to estimate the pyrolysis product of polyethylene pyrolysis at any intermediate temperature or at reasonable extrapolated temperatures. The experimental data was available only up to 850°C, but the extrapolations to 1000°C were constrained in detail to conform to mass, carbon, hydrogen, and oxygen balance.
Fig. 5 also shows extrapolations to 6000°C that may be of interest if one goes to very high temperatures—by plasma torch heating, for example. Here, we incorporate a conjecture that at the highest temperatures, carbon and hydrogen emerge among the products at the expense of the C1-C2 compounds, as well as aromatics and PNAs components.
Although we have already found that an ASEM can begin to bring some order and overview into pyrolysis yields, clearly, we have a long way to go. When the time dimension is important, the overall search is for a reasonable function of seven variables: [H], [O], a, b, c, T, and t. Einstein’s special relativity dealt with only four variables: x, y, z, and t.
SW-IGCC VS NGCC AND ACE
Before World War II almost every town had its own gas works, mainly using coal, as a feedstock. After World War II cheap NG became available and became a major PES for home heating and cooking, as well as for industrial purposes. In the 1980s factory-produced NGCC became available, and NG became a baseload fuel source for many electric utilities, hastening the drawdown of U.S. domestic supplies. In the past four years NG prices have risen to some three to seven times greater than they were when most of these NGCC facilities were built. Thus, pursuing SWEATT is very timely. For most biomass and plastic feedstock, pyrolysis is substantially equivalent to gasification.
The economic feasibility of using a gasifier in front of a gas-fired system can be examined with simple arithmetic and algebra using an analytical cost estimation (ACE) method.[6-10] Analytical cost estimation takes advantage of the almost-linear relationship shown in many detailed cost analyses of the cost of electricity (COE = Y) vs the cost of fuel (COF = X) for many technologies, i.e.,
Y(X) = K + XS (6)
Here, Y is given is in cents/kWh, and X is given in dollars/MMBtu. In Eq. 6, S is the slope of the Y(X) line in cents/kWh/$/MMBtu or 10,000 Btu/kWh. S relates to the net plant heat rate (NPHR) via
S = NPHR/10,000 or efficiency via
5 = 34.12/Eff (7)
For modern coal plants S ~ 1, although supercritical pulverized coal (SCPC) plants are reaching toward 0.9 [6-10]. Essentially, the parameter K = COE if the fuel comes to the utility without cost.
In previous studies,[6-10] we assigned Kng = 2 as a reasonable zero fuel cost parameter for, say, a 100 MW NGCC system.[32,33] This low number reflects the low capital costs of the factory-produced gas turbines and steam turbines in NGCC systems reasonable om and en costs. A slope Sng = 0.7 is a reasonable assignment reflecting the high efficiency of recent NGCC facilities.
Fig. 5 Yields vs temperature for polyethylene in various hydrocarbon groups.
For a SW integrated gasification combined cycle (SW-IGCC) system, Ksw generally would be higher than Kng because the capital costs and operating cost must include the gasifier and gas cleanup system. The value of Ssw is also higher than Sng because we must first make an SES producer gas, syngas, or pyrogas,which involves some conversion losses. Ssw= 1 is a reasonable ballpark slope for an up-to-date SW-IGCC system. The Xsw for a SW-IGCC system that would compete with a NGCC system at various Xng must satisfy
By algebra, it follows that the SW fuel cost Xsw that would enable a SWCC system to deliver electricity at the same cost as a NGCC system paying Xng is given by
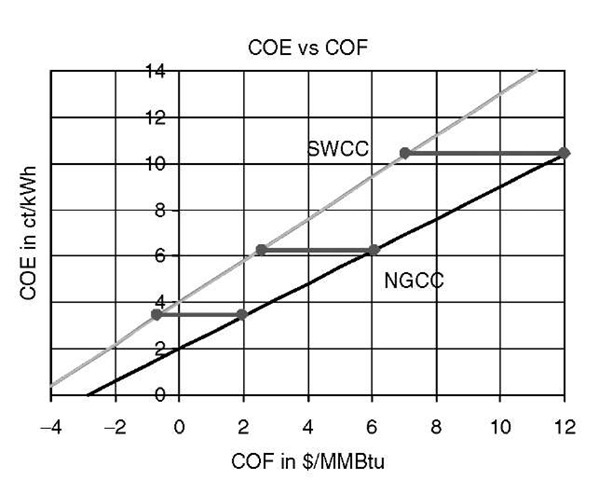
In what follow, all X numbers are in dollars/MMBtu, and all Y and K numbers are in cents/kWh. Let us use Eq. 9 with Kng = 2, Sng = 0.7, Ssw = 1 and Ksw = 4 as a reasonable ballpark numbers based on several SWCC analyses.[6-10] Then the first term in Eq. 9 is — 2. Now when the Xng = 2 to generate SWCC electricity at the same cost, the SW provider must deliver the fuel at a negative price—i.e., pay the tipping fee — 0.7. If Xng is near 6, however, as it was in 2004 and in the spring and summer of 2006, the SWCC utility could pay up to 2.4 for the SW fuel. If the Xng is 12, the SWCC facility could pay 7.1 to the SW supplier. This Xsw price is much higher than that of coal, the delivered price (Xc) of which these days usually is in the 2-3 range. This simple cost comparison is illustrated in Fig. 6, which shows the opportunities for SWCC systems when NG prices are above, say, $5/MMBtu. The results are slightly less favorable if the Ksw were higher—say, K = 5.
The conclusion, however, that at high NG prices SWCC electricity becomes competitive with NGCC electricity would be similar. It is conceivable that Ksw could be held as low as 2 cents/kWh by retrofitting a NGCC system stranded by high NG prices. In this case, the first term in Eq. 9 vanishes, and the competitive Xsw = (SngISsw)Xng. This illustrates the main point that at high NG prices with an ATT system, SW can be a valuable PES. Indeed, this simple algebraic exercise establishes the feasibility of a new paradigm in which SW (mostly biomass but here meaning all solids that are now wasted) becomes a potentially valuable marketable asset.
As described above, the values of K and S are the key factors in determining the COFsw to be used in a SW-IGCC that would be competitive on a COE basis with the COE using a NGCC system at the available COFng. The ACE method can be extended to the use of SW or biomass with other technologies if we can identify the K and S for each technology.
Fig. 6 COE vs COF for SWCC and NGCC at Xng= 2, 6, 12.
The CCTL has applied the ACE method to a large body of COE-vs-COF calculations on biomass use presented in an Antares Group, Inc. report (AGIR)[34] for several technologies. It is reasonable to apply these results to most of the SW listed in Table 1, particularly in small communities that have recycling programs involving residential separation of waste that would minimize the cost of making RDF.
The technologies investigated in the AGIR when 100 tons per day forest thinning was available include a biomass-integrated gasifier combined cycle (B-IGCC) system, a B-IG simple cycle (B-IGSC) system, a BIG internal combustion (B-IGIC) system, a biomass-gasifica-tion-coal cofiring BIG-CC of system, a direct co-firing of biomass and coal in a coal-steam boiler BCoSt, a direct use of biomass in a feedwater heat recovery arrangement (FWHR), direct use of biomass in a stoker fire boiler steam turbine (SFST) system. and direct firing in a combined heat and power plant (CHP) with a steam market at $6/ MMBtu. For each technology, it was possible to approximately represent the tabulated COE vs COF in Eq. 6 and to evaluate K and S. Then, using reasonable analytical forms for K{P) and S{P), one can make COE comparisons at various power levels and fuel costs. The most interesting result of this ACE digest of the massive AGIR tables is the fact that with slight extrapolations to higher power levels, the results in several important cases were opposite those for lower power levels. Because the assumed 100 tons per day of forest residue was rather low for many areas, these changes in conclusions were important.
Thus far, we have focused on the competition between NG-fueled technologies and SW-fueled technologies. Competition of SW-generated electricity with coal-steam-generated electricity appears to be a bigger problem. If one includes the more expensive scrubber cost in the Ks and externality cost in the coal X’s,[35] however, the SW-IGCC route should fare well. Coal burning is a major issue in many communities, yet when one projects technology directions around the globe, it is clear that the Gasification Age is returning.[36]
COMPONENTS OF ACE (CACE)
The CCTL is in the process of examining other economic COE-vs-COF analyses to quantify a more detailed formulation of ACE in which K is broken into components K=Kc + ^om + Ken, where c stands for capital costs, om for operating and maintenance costs, and en for environmental costs. At this time, establishing the magnitudes of these components for various technologies and power levels is at the cutting edge of utility economic analyses, and there are large disagreements—particularly on Ken. Future fuel costs (X) in the COE term XS in many important cases, however, probably represents the greatest source of uncertainty. Accordingly, it is foolish to belabor estimating K factors with great precision when X can range over wide limits. In this component form of ACE, Eq. 6 is replaced by
where Kcl, Koml, Kel, and are established on the basis of a detailed analysis at a reference power level Pr, and a, j, and 8 are scaling parameters intended to reflect the tendency of per-energy-unit cost to go down as the power goes up (economy of scale). These scaling parameters might be established on the basis of a broad set of studies for each technology.
Table 5 lists CACE parameters extracted from a detailed analysis, “Options for Meeting the Electrical Supply Needs of Gainesville,” prepared by ICF Consult-ing.[35] Here, the final cost of electricity is given in 2003 cents/kWh. The third case, NGCCc, has been added to allow for the contingency that with new offshore drilling or the increased development of liquefied natural gas (LNG) importing capabilities, NG might return to the $4/ MMBtu level of 2003.
Table 5 CACE applied to the ICF consulting study
| Tech | Pr | K„ | ^om | Ken | Xr($/ MMBtu) | S0 | COEr(ct/kWh) |
| NGCCa | 220 | 0.598 | 0.234 | - 0.170 | 6.10 | 0.68 | 4.81 |
| NGCCb | 220 | 0.598 | 0.234 | - 0.170 | 11.34 | 0.68 | 8.37 |
| NGCCc | 220 | 0.598 | 0.234 | - 0.170 | 4.00 | 0.68 | 3.38 |
| SCPC | 800 | 1.491 | 0.299 | 0.714 | 1.91 | 0.93 | 4.28 |
| CFB-CB | 220 | 2.531 | 0.261 | 0.618 | 1.41 | 1.05 | 4.89 |
| CFB-B | 75 | 2.845 | 0.261 | 0.039 | 1.67 | 1.39 | 5.47 |
| IGCC | 220 | 2.2 | 0.196 | 0.407 | 1.41 | 0.86 | 4.02 |
The final column shows that at the reference power levels without the NGCCs case, the IGCC scores the lowest COE, as the ICF report (ICFR) concluded. The value of the ACE analysis is that with a bit of algebra, anyone can easily consider other fuel cost projections and other power levels (with assumed values of a, /3, y, and 8). Based on previous CCTL exploratory work and economy-of-scale investigations, the author estimates that for costly field-erected facilities, a = /3 = 0.3 are reasonable choices. With factory fabrication of jet and steam turbines, however, these parameters may not follow the usual economy-of-scale pattern and be somewhat smaller in magnitude. Assigning a value for y is a wide-open question because environmental costs and the methods of incorporating them into the cost of electricity are highly debated issues.[36] Reasonable values for 8 are also somewhat difficult to find. For NGCCs, the author tentatively assigns close to zero or a very small value (~ 0.1), perhaps because the development of highly efficient aeroderivative turbines has proceeded on a wide range of power levels.
In concluding this section, it should be clear that the age of making gas has returned and that the time has come to develop a national gas strategy[37] to facilitate the earliest implementation of new gasification systems.
BIOFUELS
Biochemical Conversion by Fermentation
Fermentation, a major form of biochemical conversion, uses bacteria in the presence of oxygen to break down biodegradable organic material into liquid fuels such as ethanol. The ethanol thrust is an extension of the commercial beer, wine, and alcohol industries’ processing of sugar- and starch-based feedstock such as corn and sugarcane. An important development,1-38-1 however, has extended these capabilities to cellulose, greatly expanding the mass of biomass that can be transformed by the fermentation route. Ethanol lends itself to conventional automotive liquid fuel storage, although at only 0.6 times the energy density of diesel or gasoline. As Brazil has demonstrated, an automotive fleet can be largely fueled by ethanol. An aircraft fleet will require a higher-energy-density fuel, however.
Biochemical Conversion by Anaerobic Digestion
In anaerobic digestion, bacteria convert biodegradable waste to methane gas, a technology that is an extension of the phenomenon of flatulence of animals.[39] Landfill gas is a product of anaerobic digestion. Although a good-quality gas can be achieved by anaerobic digestion, escaping methane can be a problem, because one molecule of methane is some 20 times more damaging as a greenhouse gas than carbon.
At this time, neither the aerobic nor the anaerobic biochemical conversion is capable of processing lignin (approximately 25 wt% of plant matter) or any plastics except biodegradable plastics. The main disadvantage of bioconversion is that the reaction times are weeks, whereas thermochemical conversion can take place in minutes. Thus, the volume required for large-scale biochemical processing is very much larger than for ATT processing. The fact that the residue from biochemical conversion can be a good feedstock for thermochemical processes[1] suggests that bioconversion and thermal conversion should work together. Estimates of SW from alcohol and methane production are included in Table 1.
Bio-Oils
The esters of vegetable oils are renewable alternative fuels that potentially can serve as direct replacements for diesel fuels in compressed ignition engines (CIE).[39] Oils from soybeans, sunflower seeds, safflower seeds, cotton seeds, peanuts, and rapeseeds, as well as used oil from restaurants, are under considerable investigation as replacements for diesel. Waste from bio-oil programs have been included in Table 1 because only the seeds of the plants are used for bio-oil crops; the rest of the plant becomes SW amenable to serving as an input of a SWEATT program.
RECYCLING AND SWEATT
Although our confrontational society has a tendency to view waste-to-energy as a threat to recycling programs, the opposite may be true. Recycling programs in a community can serve to sort the various components of municipal or institutional SW into categories that lend themselves to maximizing the return on these components. If, for example, newspaper at a given time has no recycling market but must be disposed of at a cost, it could be used as high-energy dry feedstock for ATTs. The same is true for plastics recycling. Thus, the marketplace would be decisive as to whether to recycle via the materials route or the energy route. A recycling community should be able to go the SW-IGCC route with less capital costs than one that does not have waste separation at the source.
The advantages of a biomass alliance with natural gas (BANG) have been described previously.[6-10] Gasification systems that mainly use cellusosic (biomass) inputs produce a low- or medium-heating-value fuel that will result in the derating of a NG designed turbine-generator. By coutilizing the biomass pyrogas with NG, one can ensure that the input energy requirement matches the output needs at least until the maximum rating of the generator is required. At that point, the firing could be entirely on NG. In a solid waste alliance with natural gas (SWANG), an additional option becomes available when the SW comes from a recycling community. Then the utility might prepare and store high-energy plastics for increased use during times of high electricity demand as a means of following peak loads without calling on the full use of NG.
ATT FOR LIQUID FUEL PRODUCTION
Pyrolysis/gasification technologies followed by gas cleanup can greatly reduce emissions of pollutants such as NOX and SOX, as well as toxics such as mercury and arsenic. Advanced thermal technologies can treat nearly the entire organic fraction of MSW and, in general, can treat a more heterogeneous feedstock, including high-energy-content plastics.[11] Although this paper focuses on gaseous fuel generation, ATTs for liquid fuel (condensable gas) production are closely related. Considerable research and development work is under way on distillation technologies to refine such liquid fuels for transportation applications, adding a major driver for the ATT route.
It should be noted that Table 1 does not list oil shale or tar sands in the United States that could substantially increase the available SW tonnage that could be used to address our need for transportation fuels. A 2005 Rand study[40] shows that with in-situ thermal treatment, domestic oil shales could substantially lower our oil import problem. Another route would be to convert our coal to liquid fuels, as South Africa has done for many years. A third route would involve the use of methane hydrates to produce methane for use in NG-fueled vehicles.
CONCLUSIONS
The main conclusion of this paper is that the United States has very large sustainable supplies of now-wasted solids that have an annual energy potential comparable to that of our current use of coal and also of NG. With ATT, this SW could, in the near term, multiply its contribution to our national energy supply by a factor of about 10. Robust technology that can handle MSW or RDF also should be able to handle almost any of the categories listed in Table 1. Agricultural and forestry residues are two of the major SW supply components in this list, and many of the other materials are greenhouse-neutral plant matter.
By utilizing thermochemical processes to convert the lignin and plastic content of SW and biochemical residues, we could get much closer to goal of zero SW. Solid waste to energy by advanced thermal technologies also would further reduce the final volume of the waste, and practically all contaminants can be destroyed by high temperatures. Thus, cooperation between biochemical and thermochemical programs would clearly be in the national interest.
Japan, a country with an outstanding sustainability record, is the global leader in SWEATT. With more than 60 pyrolysis and thermal gasification systems now in operation, Japan has established the technical and environmental feasibility of these systems. This should allay the concerns of environmentalists and risk-averse utility decision makers in the United States. In final summary, our main conclusions are
• The United States is excessively reliant on imported oil (60%) for its liquid fuels.
• The United States is increasingly reliant on imported NG fuel (now > 15%).
• The United States is well endowed with solid fuels: wasted solids, coal, and oil shale.
• The organic matter in SW can be converted to useful gaseous or liquid fuels.
• In most cases, ATT provides the fastest and most efficient conversion method.
• Co-use of domestic fuels such as SW and NG can overcome some problems.
• Solid waste to energy by advanced thermal technologies generates lower emissions than combustion waste-to-energy systems.
• Thermal conversion of solid fuels to gaseous and liquids fuels has a long history.
• Utilities are experienced with high-temperature processes in the production of steam.
• Advanced thermal technologies (PABC, POBC, and PYRO) are extensions of high-temperature steam making.
• Conversion to gaseous fuels is essential for SW powering of fuel cells.
• There are many environmental benefits attendant to SWEATT.
• Many areas of engineering research will be needed to optimize SWEATT.
• Cooperation of stakeholders would accelerate the implementation of ATT.
• Conservation and SWEATT together is the fastest realistic path to zero waste.
Glossary
ABPC: Air Blown Partial Combustion ACE: Analytical Cost Estimation AGIR: Antares Group Inc. Report Ar: Aromatics
ASEM: Analytical Semiempirical Model
ATT: Advanced Thermal Technologies
BTU: British Thermal Units
CACE: Component Analytic Cost Estimation
CCTL: Clean Combustion Technologies Laboratory
CHP: Combined Heat And Power
CIE: Compressed Ignition Engines
DANSF: Dry Ash, Nitrogen And Sulfur Free
EU: European Union
FC: Fixed Carbon
GT: Gas Turbines
HHV: Higher Heating Values
HRSG: Heat Recovery Steam Generator
ICE: Internal Combustion Engines
IGCC: Integrated Gasifier Combined Cycle
MSW: Municipal Solid Waste
NGCC: Natural Gas-Fired Combined Cycle
NPHR: Net Plant Heat Rate
OBPC: Oxygen Blown Partial Combustion
OFC: Omnivorous Feedstock Converter
PES: Primary Energy Supplies
PNA: Polynuclear Aromatics
PYRO: Pyrolysis Systems
QES: Quaternary Energy Supply
quads: Quadrillion BTUs
RDF: Refuse Derived Fuels
SES: Secondary Energy Supplies
SW: Solid Waste
SWANG: Solid Waste Alliance with Natural Gas
SWEATT: Solid Waste To Energy By Advanced Thermal
Technologiess TES: Tertiary Energy Supply TPES: Total Primary Energy Supply VT: Volatiles
WEC: Waste to Energy Conversion
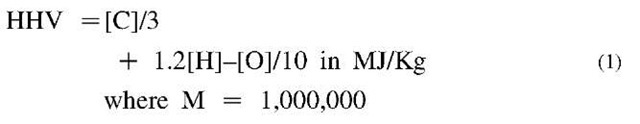
![(A) Weight percentages of hydrogen [H] vs [O] for 185 DANSF carbonaceous materials (black diamonds) vs oxygen wt%. Classification labels are given at the bottom scale and [O] values on top scale. Adapted from Ref. [4]. (B) Higher heating values (HHV) of 185 carbonaceous materials (corrected to DANSF) vs [O]. The smooth curve represents HHV= ([C]/3+ 1.2[H]-[O]/10). (C) Total volatile weight percentages vs [O] for 185 DASNF carbonaceous materials (squares) from proximate analysis. The curve through the data points satisfies VT = 62([H]/6)([O]/25)1/2. The analytic fixed carbon (FC) is shown. (A) Weight percentages of hydrogen [H] vs [O] for 185 DANSF carbonaceous materials (black diamonds) vs oxygen wt%. Classification labels are given at the bottom scale and [O] values on top scale. Adapted from Ref. [4]. (B) Higher heating values (HHV) of 185 carbonaceous materials (corrected to DANSF) vs [O]. The smooth curve represents HHV= ([C]/3+ 1.2[H]-[O]/10). (C) Total volatile weight percentages vs [O] for 185 DASNF carbonaceous materials (squares) from proximate analysis. The curve through the data points satisfies VT = 62([H]/6)([O]/25)1/2. The analytic fixed carbon (FC) is shown.](http://lh3.ggpht.com/_1wtadqGaaPs/TGriAJ5sbQI/AAAAAAAARe0/UHy0dwwkoYM/tmp2867_thumb_thumb.jpg?imgmax=800)
![(A) Weight percentages of hydrogen [H] vs [O] for 185 DANSF carbonaceous materials (black diamonds) vs oxygen wt%. Classification labels are given at the bottom scale and [O] values on top scale. Adapted from Ref. [4]. (B) Higher heating values (HHV) of 185 carbonaceous materials (corrected to DANSF) vs [O]. The smooth curve represents HHV= ([C]/3+ 1.2[H]-[O]/10). (C) Total volatile weight percentages vs [O] for 185 DASNF carbonaceous materials (squares) from proximate analysis. The curve through the data points satisfies VT = 62([H]/6)([O]/25)1/2. The analytic fixed carbon (FC) is shown. (A) Weight percentages of hydrogen [H] vs [O] for 185 DANSF carbonaceous materials (black diamonds) vs oxygen wt%. Classification labels are given at the bottom scale and [O] values on top scale. Adapted from Ref. [4]. (B) Higher heating values (HHV) of 185 carbonaceous materials (corrected to DANSF) vs [O]. The smooth curve represents HHV= ([C]/3+ 1.2[H]-[O]/10). (C) Total volatile weight percentages vs [O] for 185 DASNF carbonaceous materials (squares) from proximate analysis. The curve through the data points satisfies VT = 62([H]/6)([O]/25)1/2. The analytic fixed carbon (FC) is shown.](http://lh6.ggpht.com/_1wtadqGaaPs/TGrifJgLhDI/AAAAAAAARe8/fQ_7tnpLsDQ/tmp2868_thumb_thumb.jpg?imgmax=800)
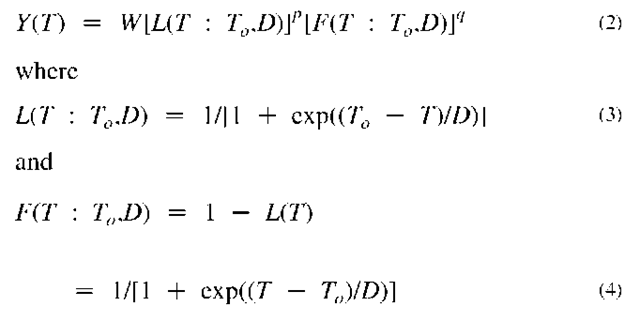
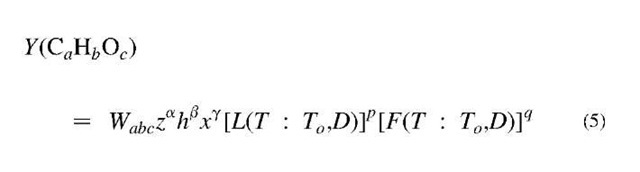
![Wt% yields vs temperature (in °C) from pyrolysis of anthracite, bituminous, lignite, and wood with ([C], [H], [O]) as shown. HC represents C2 and C3 gasses, BTX, phenol and cresol. Wt% yields vs temperature (in °C) from pyrolysis of anthracite, bituminous, lignite, and wood with ([C], [H], [O]) as shown. HC represents C2 and C3 gasses, BTX, phenol and cresol.](http://lh5.ggpht.com/_1wtadqGaaPs/TGrjsTnp73I/AAAAAAAARfk/fKQiO0X54Zc/tmp22972_thumb1.jpg?imgmax=800)