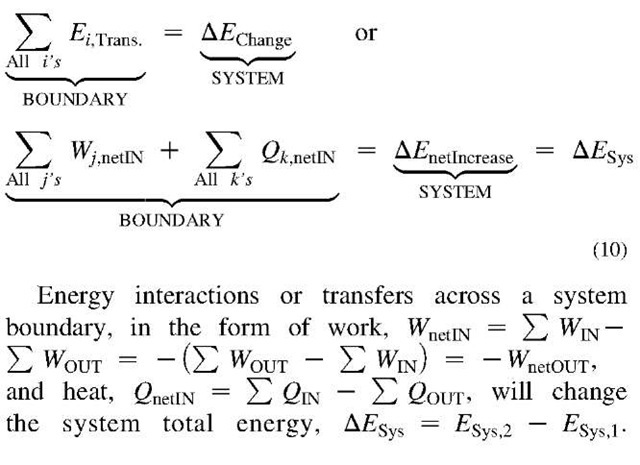
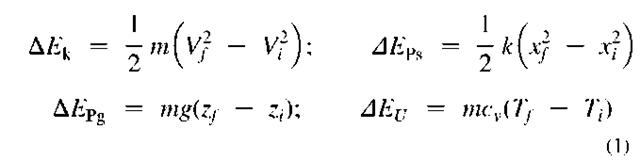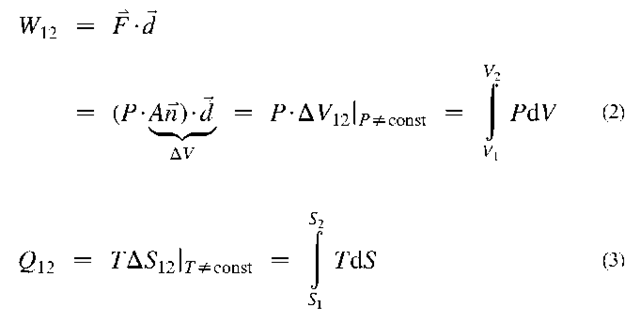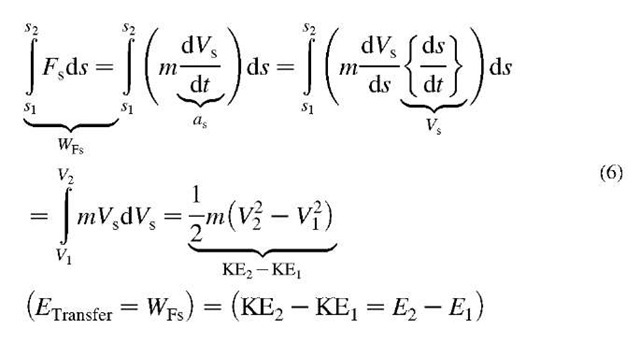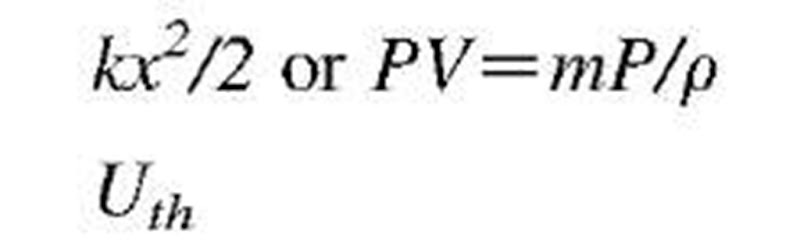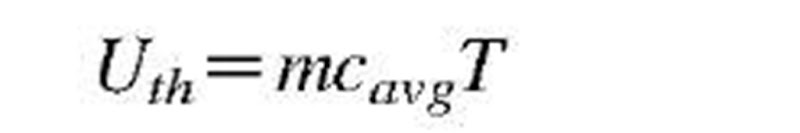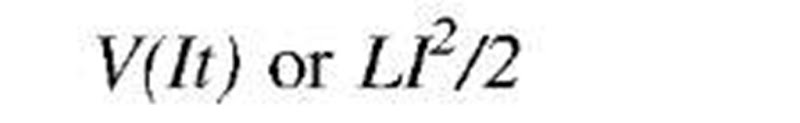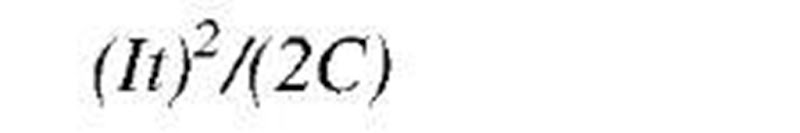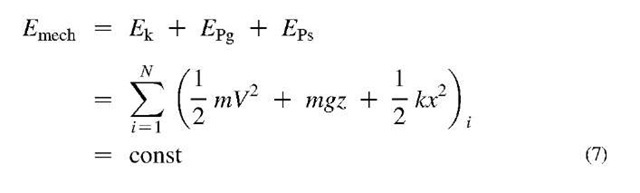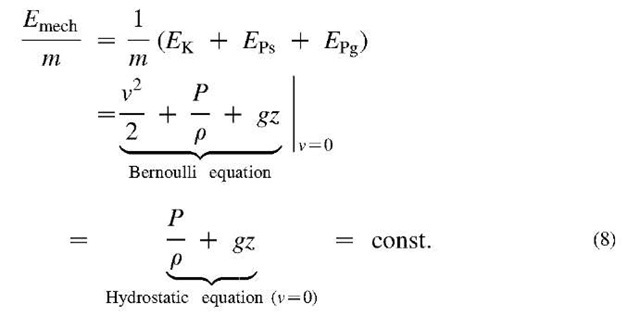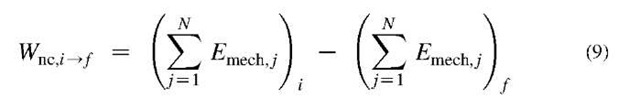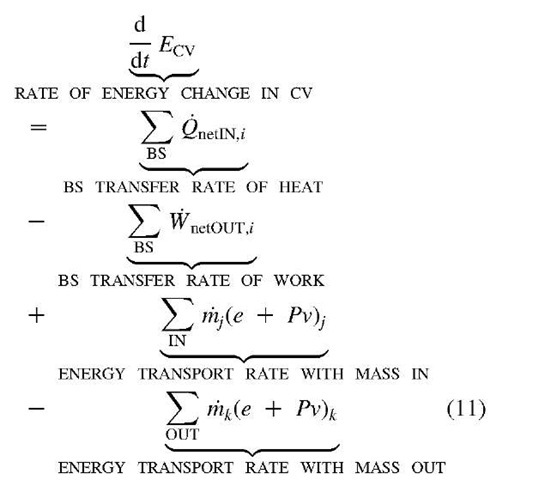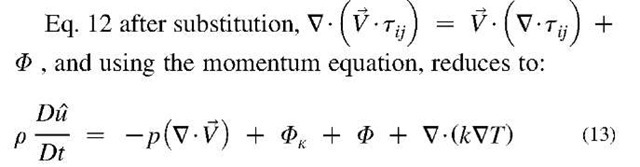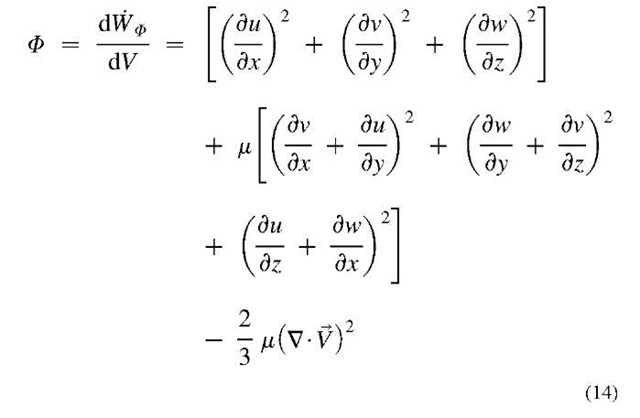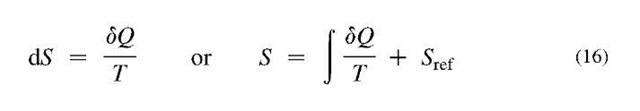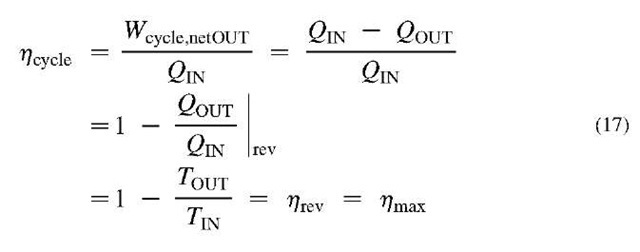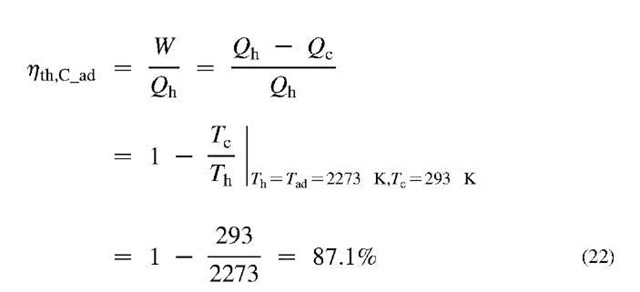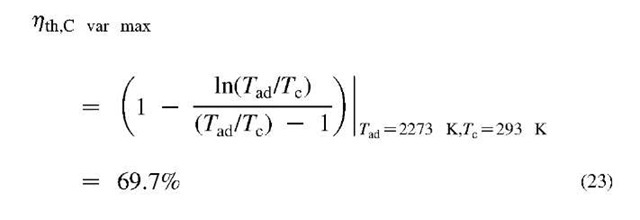Abstract
The concept and definition of energy are elaborated, as well as different forms and classification of energy are presented. Energy is a fundamental concept indivisible from matter and space, and energy exchanges or transfers are associated with all processes (or changes), thus indivisible from time. Actually, energy is “the building block” and fundamental property of matter and space, thus fundamental property of existence. Any and every material system in nature possesses energy. The structure of any matter and field is “energetic,” meaning active, i.e., photon waves are traveling in space, electrons are orbiting around an atom nucleus or flowing through a conductor, atoms and molecules are in constant rotation, vibration or random thermal motion, etc. When energy is exchanged or transferred from one system to another it is manifested as work or heat. In addition, the First Law of energy conservation and the Second Law of energy degradation and entropy generation are presented along with relevant concluding remarks. In summary, energy is providing existence, and if exchanged, it has ability to perform change.
INTRODUCTION: FROM WORK TO HEAT TO GENERAL ENERGY CONCEPT
Energy is a fundamental concept indivisible from matter and space, and energy exchanges or transfers are associated with all processes (or changes), thus indivisible from time. Actually, energy is “the building block” and fundamental property of matter and space, thus a fundamental property of existence. Energy transfer is needed to produce a process to change other properties. Also, among all properties, energy is the only one which is directly related to mass and vice versa: E = mc2 (known in some literature as mass energy, the c is the speed of light in a vacuum), thus the mass and energy are interrelated.[1,2] Energy moves cars and trains, and boats and planes. It bakes foods and keeps them frozen for storage. Energy lights our homes and plays our music. Energy makes our bodies alive and grow, and allows our minds to think. Through centuries, people have learned how to use energy in different forms in order to do work more easily and live more comfortably. No wonder that energy is often defined as ability to perform work, i.e., as a potential for energy transfer in a specific direction (displacement in force direction) thus achieving a purposeful process, as opposed to dissipative (less-purposeful) energy transfer in form of heat. The above definition could be generalized as: energy is providing existence, and if exchanged, it has the ability to perform changed3]
Any and every material system in nature possesses energy. The structure of any matter and field is “energetic,” meaning active, i.e., photon waves are traveling in space, electrons are orbiting around an atom nucleus or flowing through a conductor, atoms and molecules are in constant rotation, vibration or random thermal motion, etc., see Table 1 and Fig. 1. Thus energy is a property of a material system (further on simply referred to as system), and together with other properties defines the system equilibrium state or existence in space and time.
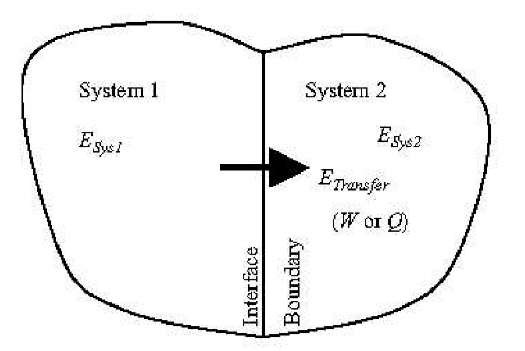
Energy in transfer (Etransfer) is manifested as work (W) or heat (Q) when it is exchanged or transferred from one system to another, as explained next (see Fig. 2).
Work
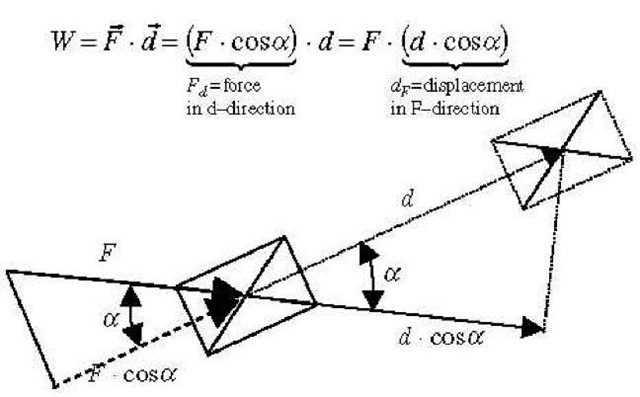
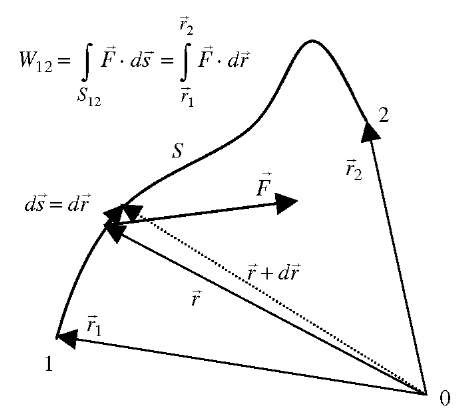
Work is a mode of energy transfer from one acting body (or system) to another resisting body (or system), with an acting force (or its component) in the direction of motion, along a path or displacement. A body that is acting (forcing) in motion-direction in time, is doing work on another body which is resisting the motion (displacement rate) by an equal resistance force, including inertial force, in opposite direction of action. The acting body (energy source) is imparting (transferring away) its energy to the resisting body (energy sink), and the amount of energy transfer is the work done by the acting onto the resisting body, equal to the product of the force component in the motion direction multiplied with the corresponding displacement, or vice versa (force multiplied by displacement component in the force direction), see Fig. 3. If the force (F) and displacement vectors (ds = dr) are not constant, then integration of differential work transfers from initial (1) to final state (2), defined by the corresponding position vectors r, will be necessary, see Fig. 4.
Table 1 Material system structure and related forces and energies
| Particles | Forces | Energies |
| Atom nucleus | Strong and weak | Nuclear |
| Electron shell, or electron flow | Electromagnetic | Electrical, magnetic, electromagnetic |
| Atoms/molecules | Inter-atomic/molecular | Chemical |
| Molecules | Inertial due to random collision and, potential inter-molecular | Sensible thermal |
| Molecules | Potential inter-molecular | Latent thermal |
| Molecules | Potential inter-molecular | Mechanical elastic |
| System mass | Inertial and gravitational | Mechanical kinetic and gravitational potential |
The work is a directional energy transfer. However, it is a scalar quantity like energy, and is distinctive from another energy transfer in form of heat, which is due to random motion (chaotic or random in all directions) and collisions of system molecules and their structural components.
Work transfer cannot occur without existence of the resisting body or system, nor without finite displacement in the force direction. This may not always be obvious. For example, if we are holding a heavy weight or pushing hard against a stationary wall, there will be no work done against the weight or wall (neglecting their small deformations). However, we will be doing work internally due to contraction and expansion of our muscles (thus force with displacement), and that way converting (spending) a lot of chemical energy via muscle work, then dissipating it into thermal energy and heat transfer (sweating and getting tired).
Heat
Heat is another mode of energy transfer from one system at higher temperature to another at lower temperature due to their temperature difference. Fire was civilization’s first great invention, long before people could read and write, and wood was the main heat source for cooking and heating for ages. However, true physical understanding of the nature of heat was discovered rather recently, in the middle of the nineteenth century, thanks to the development of the kinetic theory of gasses. Thermal energy and heat are defined as the energy associated with the random motion of atoms and molecules. The prior concept of heat was based on the caloric theory proposed by the French chemist Antoine Lavoisier in 1789. The caloric theory defines heat as a massless, colorless, odorless, and tasteless, fluid-like substance called the caloric that can be transferred or “poured” from one body into another. When caloric was added to a body, its temperature increased and vice versa. The caloric theory came under attack soon after its introduction. It maintained that heat is a substance that could not be created or destroyed. Yet it was known that heat can be generated indefinitely by rubbing hands together or from mechanical energy during friction, like mixing or similar. Finally, careful experiments by James P. Joule published in 1843, quantified correlation between mechanical work and heat, and thus put the caloric theory to rest by convincing the skeptics that heat was not the caloric substance after all. Although the caloric theory was totally abandoned in the middle of the nineteenth century, it contributed greatly to the development of thermodynamics and heat transfer.
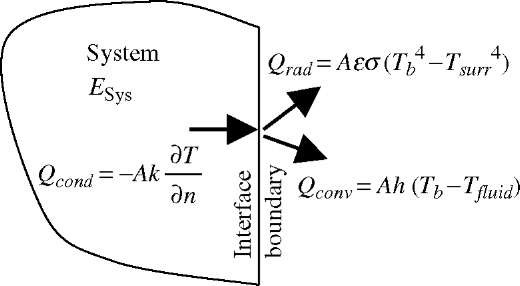
Heat may be transferred by three distinctive mechanisms: conduction, convection, and thermal radiation, see Fig. 5. Heat conduction is the transfer of thermal energy due to interaction between the more energetic particles of a substance, like atoms and molecules (thus at higher temperature), to the adjacent less energetic ones (thus at lower temperature). Heat convection is the transfer of thermal energy between a solid surface and the adjacent moving fluid, and it involves the combined effects of conduction and fluid motion. Thermal radiation is the transfer of thermal energy due to the emission of electromagnetic waves (or photons) which are products of random interactions between energetic particles of a substance (thus related to the temperature). During those interactions the electron energy level is changed, thus causing emission of photons, i.e., electromagnetic thermal radiation.
The Joule’s experiments of establishing equivalency between work and heat paved the way of establishing the concept of internal thermal energy, to generalize the concept of energy, and to formulate the general law of energy conservation. The total internal energy includes all other possible but mechanical energy types or forms, including chemical and nuclear energy. This allowed extension of the well-established law of mechanical energy conservation to the general law of energy conservation, known as the First Law of Thermodynamics, which includes all possible energy forms that a system could possess, and heat and all types of work as all possible energy-transfers between the systems. The Law of Energy Conservation will be elaborated later.
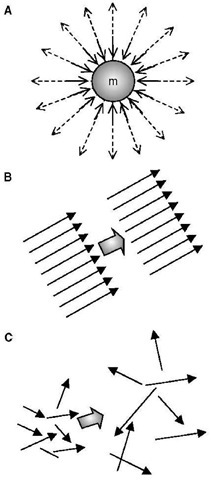
Fig. 1 Different types of energy: (A) Potential gravitational and electromagnetic radiation; (B) Organized energy as work transfer; (C) Disorganized thermal energy as heat transfer.
Energy
Energy is fundamental property of a physical system and refers to its potential to maintain a system identity or structure and to influence changes with other systems (via forced interaction) by imparting work (forced directional displacement) or heat (forced chaotic displacement/motion of a system molecular or related structures). Energy exists in many forms: electromagnetic (including light), electrical, magnetic, nuclear, chemical, thermal, and mechanical (including kinetic, elastic, gravitational, and sound); where, for example, electro-mechanical energy may be kinetic or potential, while thermal energy represents overall potential and chaotic motion energy of molecules and/or related micro [3 41 structure.
Fig. 2 Energy as material system property and energy transfer from one system to another.
Energy, Work, and Heat Units
Energy, Work, and Heat Units. Energy is manifested via work and heat transfer, with corresponding Force X Length dimension for work (N m, kgfm, and lbft, in SI, metric and English system of units, respectively); and the caloric units, in kilocalorie (kcal) or British-thermal-unit (Btu), the last two defined as heat needed to increase a unit mass of water (at specified pressure and temperature) for one degree of temperature in their respective units. Therefore, the water specific heat is 1 kcal/(kg °C) = 1 Btu/(lb °F) by definition, in metric and English system of units, respectively. It was demonstrated by Joule that 4187 N m of work, when dissipated in heat, is equivalent to 1 kcal. In his honor, 1 N m of work is named after him as 1 Joule, or 1 J, the SI energy unit, also equal to electrical work of 1 Ws = 1 VAs. The SI unit for power, or work rate, is watt, i.e., 1 J/s = 1 W, and also corresponding units in other system of units, like Btu/h, etc. The Horse Power is defined as 1 HP = 550 lbfft/s = 745.7 W. Other common units for energy, work and heat are given in Table 2.
Fig. 3 Work, force, and displacement.
Fig. 4 Work along an arbitrary path.
ENERGY FORMS AND CLASSIFICATIONS: ENERGY-TRANSFER VS ENERGY-PROPERTY
Any and all changes or processes (happening in space and time) are caused by energy exchanges or transfers from one substance (system or subsystem) to another, see Fig. 2. A part of a system may be considered as a subsystem if energy transfer within a system is taking place, and inversely, a group of interacting systems may be considered as a larger isolated system, if they do not interact with the rest of the surroundings. Energy transfer may be in organized form (different types of work transfer due to different force actions) or in chaotic disorganized form (heat transfer due to temperature difference). Energy transfer into a system builds up energy-potential or generalized-force (called simply potential for short, like pressure, temperature, voltage, etc.) over energy—displacement (like volume, entropy, charge, etc.). Conversely, if energy is transferred from a system, its energy potential is decreased. That is why net-energy is transferred from higher to lower energy potential only, due to virtually infinite probability of equi-partition of energy over system micro-structure, causing system equilibrium, otherwise virtually impossible singularity of energy potential at infinite magnitude would result.[4]
There are many forms and classifications of energy, see Table 3, all of which could be classified as microscopic (or internal within a system microstructure) and/or macroscopic (or external as related to the system mass as a whole with reference to other systems). Furthermore, energy may be “quasi-potential” (associated with a system equilibrium state and structure, i.e., system property) or “quasi-kinetic” (energy in-transfer from one system or one structure to another, in form of work or heat).
Fig. 5 Heat as energy-transfer by conduction, convection, and radiation is due to a difference in temperature.
Every material system state is an equilibrium potential “held” by forces (space force-fields), i.e., the forces “define” the potential and state—action and reaction; otherwise a system will undergo dynamic change (in time), or quasi-kinetic energy exchange towards another stable equilibrium. Atoms (mass) are “held” by atomic and electromagnetic forces in small scale and by gravity in large scale, see Fig. 1A, otherwise mass would disintegrate (“evaporate” or radiate into energy) like partly in nuclear reactions—nuclear energy or electromagnetic radiation. Molecules are “held” by electro-chemical bonding (valence) forces (chemical reactions—chemical energy). Liquids are “held” by latent intermolecular forces (gas condensation, when kinetic energy is reduced by cooling—latent thermal energy). Solids are “held” by “firm” intermolecular forces (freezing/solidification when energy is further reduced by cooling—latent thermal energy again). Sensible thermal energy represents intermolecular potential energy and energy of random molecular motion, and is related to temperature of a system. “Holding” potential forces may be “broken” by energy transfer (e.g., radiation, heating, high-energy particles interactions, etc.). States and potentials are often “hooked” (i.e., stable) and thus need to be “unhooked” (or to “be broken”) to overcome existing “threshold” or equilibrium, like in igniting combustion, starting nuclear reaction, etc.
As stated above, energy transfer can be directional (purposeful or organized) and chaotic (dissipative or disorganized). For example, mass-in-motion, mechanical kinetic energy, and electricity-in-motion, electrical kinetic energy, are organized kinetic energies (Fig. 1B), while thermal energy is disorganized chaotic energy of motion of molecules and atoms (Fig. 1C). System energy may be defined with reference to position in a vector-force field, like elastic potential (stress) energy, gravitational potential energy, or electromagnetic field energy. There are many different energy forms and types (see Table 3). We are usually not interested in (absolute) energy level, but in the change of energy (during a process) from an initial state (i) to a final state f), and thus zero reference values for different energy forms are irrelevant, and often taken arbitrarily for convenience. The followings are basic correlations for energy changes of several typical energy forms, often encountered in practice: motion kinetic energy (EK=KE) as a function of system velocity (V) potential elastic-deformational, e.g., pressure elastic or spring elastic energy (EPdeff = EPp = EPs) as a function of spring deformation displacement (x); gravitational potential energy (EPg = PEg) as a function of gravitational
elevation (z); and sensible thermal energy (Ev = U) as a function of system temperature (I):
Table 2 Typical energy units with conversion factors
| Energy units | J | kWh | Btu |
| 1 Joule (J) | 1 | 2.78 X10″ 7 | 9.49 X10″ 4 |
| 1 Kilowatt-hour (kWh) | 3.6 X106 | 1 | 3.412X 103 |
| 1 Kilocalorie (kcal = Cal= 1000 cal) | 4187 | 1.19 X10″ 3 | 3.968 |
| 1 British thermal unit (Btu) | 1055 | 2.93 X10″ 4 | 1 |
| 1 Pound-force foot (lbf ft) | 1.36 | 3.78 X10″ 7 | 1.29 X10″ 3 |
| 1 Electron volt (eV) | 1.6 X10″19 | 4.45 X10″ 26 | 1.52 X10″ 22 |
| 1 Horse Power X second (HP sec) | 745.7 | 2.071 X10″ 4 | 0.707 |
If the reference energy values are taken to be zero when the above initial (/) variables are zero, then the above equations will represent the energy values for the final values (f) of the corresponding variables. If the corresponding parameters, spring constant k, gravity g, or constant-volume specific heat cv, are not constant, then integration of differential energy changes from initial to final state will be necessary.
Energy transfer via work W (net-out), and heat transfer Q (net-in), may be expressed for reversible processes as product of related energy-potentials (pressure P, or temperature T) and corresponding energy-displacements (change of volume V and entropy S, respectively), i.e.:
Note, in Eq. 2, that force cannot act at a point but is distributed as pressure (P) over some area A (with orthogonal unit vector it), which when displaced will cause the volume change AV. Also note that it is custom in some references to denote heat transfer “in” and work transfer “out” as positive (as they appear in a heat engine). In general, “in” (means “net-in”) is negative “out” (means “net-out”) and vice versa.
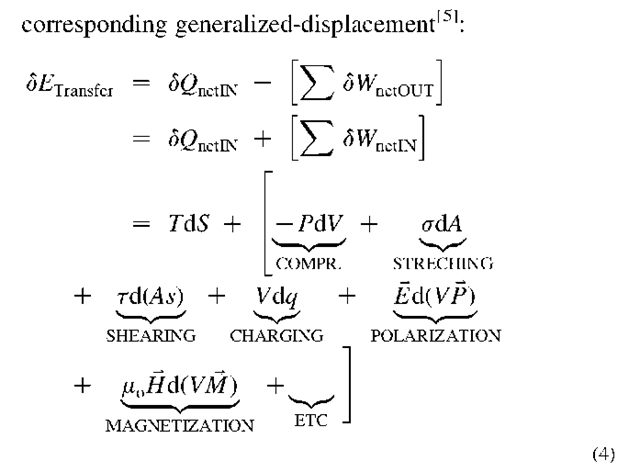
In general, energy transfer between systems is taking place at the system boundary interface and is equal to the product of energy-potential or generalized-force and the
Where the quantities after the last equal sign are: temperature and entropy; pressure and volume; surface tension and area; tangential-stress and area with tangential-displacement, voltage and electrical charge; electric field strength and volume-electric dipole moment per unit volume product; and permeability of free space, magnetic field strength and volume-magnetic dipole moment per unit volume product; respectively.
The total system energy stored within the system, as energy property, is:
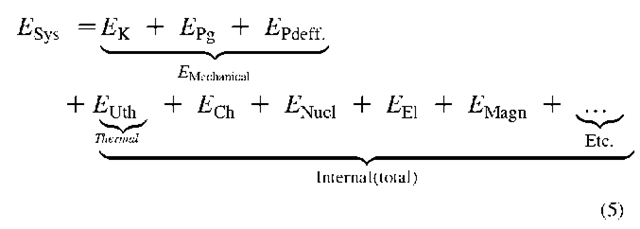
Where the quantities after the equal sign are: kinetic, potential-gravitational, potential-elastic-deformational, thermal, chemical, nuclear, electric, magnetic energies, etc.
THE FIRST LAW OF ENERGY CONSERVATION: WORK-HEAT-ENERGY PRINCIPLE
Newton formulated the general theory of motion of objects due to applied forces (1687). This provided for concepts of mechanical work, kinetic and potential energies, and development of solid-body mechanics. Furthermore, in absence of non-mechanical energy interactions, excluding friction and other dissipation effects, it is straightforward to derive (and thus prove) energy conservation, i.e.:
Table 3 Energy forms and classifications
| ENERGY | |||||
| Transfer | |||||
| Type | (release) | ||||
| Scale | Kinetic (motion) | Kinetic (motion) | |||
 |
Energy form (Energy storage) | Energy process |  |
 |
 |
The above correlation is known as the work-energy principle. The work-energy principle could be easily expended to include work of gravity force and gravitational potential energy as well as elastic spring force and potential elastic spring energy.[3]
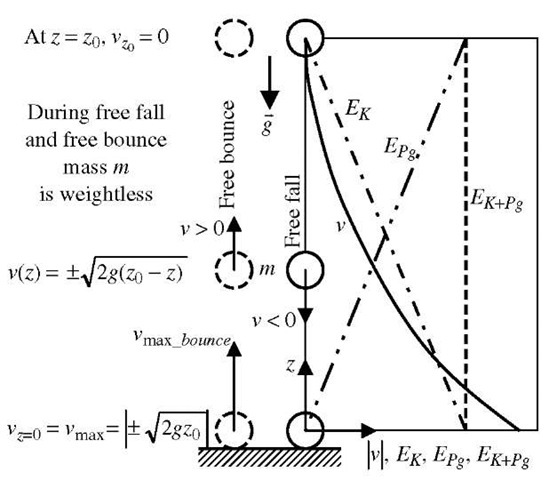
During a free gravity fall (or free bounce) without air friction, for example, the potential energy is being converted to kinetic energy of the falling body (or vice versa for free bounce), and at any time the total mechanical energy (sum of kinetic and potential mechanical energies) is conserved, i.e., stays the same, see Fig. 6. The mechanical energy is also conserved if a mass freely vibrates on an ideally elastic spring, or if a pendulum oscillates around its pivot, both in absence of dissipative effects, like friction or non-elastic deformation. In general, for work of conservative forces only, the mechanical energy, £mech , for N isolated systems, is conserved since
Fig. 6 Energy and work due to conservative gravity force.
there is no dissipative conversion into thermal energy and thus no heat transfer, i.e.:
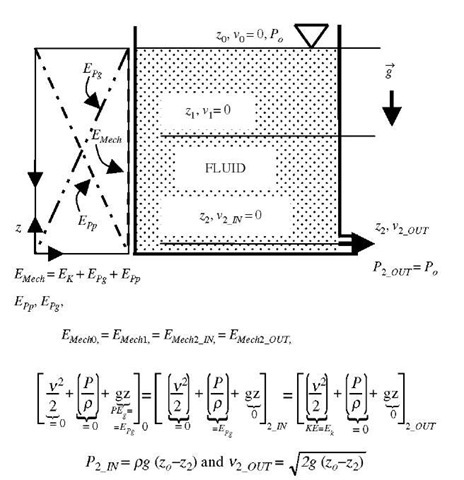
The mechanical work-energy concept could also be expended to fluid motion by inclusion of elastic-pressure force and potential elastic-pressure energy (referred to in some references as flow work; however, note that elastic-pressure energy is a system property while the flow work is related energy transfer), see the Bernoulli equation below. For flowing or stationary fluid without frictional effects, the mechanical energy is conserved, including fluid elastic—pressure energy, PV = mPIp (where V is volume, whereas v is used for velocity here), as expressed by the Bernoulli or hydrostatic equations below, see also Fig. 7.[3]
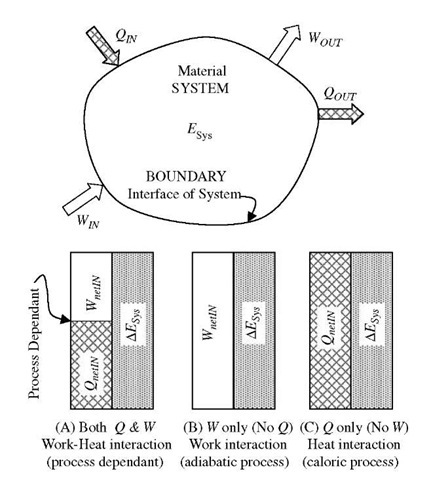
Work against inertial and/or conservative forces (also known as internal, or volumetric, or space potential field), is path-independent and during such a process the mechanical energy is conserved. However, work of non-conservative, dissipative forces is process path-dependent and part of the mechanical energy is converted (dissipated) to thermal energy, see Fig. 8A.
Fig. 7 Conservation of fluid mechanical energy: Bernoulli equation, hydrostatic equation, and Torricelli’s orifice velocity.
Fig. 8 System energy and energy boundary interactions (transfers) for (A) arbitrary, (B) adiabatic, and (C) caloric processes.
When work of non-conservative forces Wnc , is exchanged between N isolated systems, from an initial (0 to final state f), then the total mechanical energy of all systems is reduced by that work amount, i.e.:
When work of non-conservative forces Wnc , is exchanged between N isolated systems, from an initial (0 to final state f), then the total mechanical energy of all systems is reduced by that work amount, i.e.:
Regardless of the traveled path (or displacement), the work against conservative forces (like gravity or elastic spring in above cases) in absence of any dissipative forces, will depend on the final and initial position (or state) only. However, the work of non-conservative, dissipative forces (Wnc) depends on the traveled path since the energy is dissipated during the force displacement, and mechanical energy will not be conserved, but in part converted (via dissipation and heat transfer) into thermal energy, see Eq. 9. This should not be misunderstood with the total energy conservation, which is always the case, and it must include both work and heat transfer, see below.
As already stated, there are many different types of work transfer into (or out of) a system which will change the corresponding energy-form stored in (or released, discharged out of) the system. In addition to work, energy may be transferred as heat caused by temperature difference and associated with change of the thermal energy of a system. Furthermore, different forms of stored energy are often coupled so that one type of energy transfer may change more than one form of stored energy, particularly due to inevitable dissipative conversion of work to heat, and in turn to internal thermal energy. Conversely, heat and thermal energy may be converted into other energy forms. In the absence of nuclear reaction (no conversion of mass into energy, E = mc2), mass and energy are conserved separately for an isolated system, a group of isolated systems, or for the Universe. Since the material system structure is of particulate form, then systems’ interactions (collisions at different scale-sizes) will exchange energy during the forced displacement— and similarly to the mechanical energy conservation—the totality of all forms of energy will be conserved,[8] see Fig. 8, which could be expressed as:
The boundary energy transfers are process (or process path) dependent for the same A£Sys change, except for special cases for adiabatic processes with work interaction only (no heat transfer), or for caloric processes with heat interaction only (no work transfer), see Fig. 8A to C. For the latter caloric processes without work interactions (no volumetric expansion or other mechanical energy changes), the internal thermal energy is conserved by being transferred from one system to another via heat transfer only, known as caloric. This demonstrates the value of the caloric theory of heat that was established by Lavoisier and Laplace (1789), the great minds of the 18th century. Ironically, the caloric theory was creatively used by Sadi Carnot to develop the concept of reversible cycles for conversion of caloric heat to mechanical work as it “flows” from high to low temperature reservoirs (1824) that later helped in dismantling the caloric theory. The caloric theory was discredited by establishing the heat equivalent of work, e.g., mechanical equivalent of heat by Mayer (1842) and experimentally confirmed by Joule (1843), which paved the way for establishing the First Law of Energy Conservation and new science of Thermodynamics (Clausius, Rankine, and Kelvin, 1850 and later). Prejudging the caloric theory now as a “failure” is unfair and unjustified since it made great contributions in calorimetry and heat transfer, and it is valid for caloric processes (without work interactions). The coupling of work-heat interactions and conversion between thermal and mechanical energy are outside of the caloric theory domain and are further developed within the First and the Second Laws of Thermodynamics.
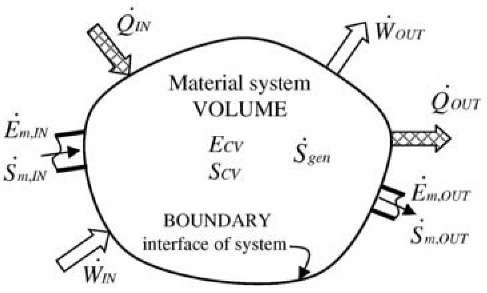
The First Law of energy conservation for the control-volume (CV, with boundary surface BS) flow process, see Fig. 9, is:
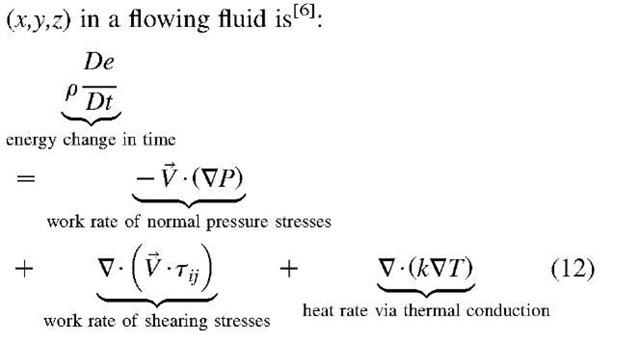
The First Law of energy conservation equation for a differential volume per unit of volume around a point
Fig. 9 Control-volume (CV) energy and entropy, and energy and entropy flows through the boundary interface of the control volume.
Where e = u + + gZ NOTE: distinguish specific thermal energy u, from velocity component u below.
Where e = u + + gZ NOTE: distinguish specific thermal energy u, from velocity component u below.
Where, $>K = k (V • v)2 is the bulk-viscosity dissipation, and $ is the shear-viscosity dissipation function, which is the rate of mechanical work conversion to internal thermal energy for a differential volume per unit of volume, with [W/m3] unit, is given for Newtonian fluid as:
The power or work rate of viscous dissipation (irreversible conversion of mechanical to thermal energy) in a control
THE SECOND LAW OF ENERGY DEGRADATION: ENTROPY AND EXERGY
Every organized kinetic energy will, in part or in whole (and ultimately in whole), disorganize/dissipate within the microstructure of a system (in time over its mass and space) into disorganized thermal energy. Entropy, as an energy-displacement system property, represents the measure of energy disorganization, or random energy redistribution to smaller-scale structure and space, per absolute temperature level. Contrary to energy and mass, which are conserved in the universe, entropy is continuously generated (increased) due to continuous redistribution and disorganization of energy in transfer and thus degradation of the quality of energy (“spreading” of energy towards and over lower potentials in time until equi-partitioned over system structure and space). Often, we want to extract energy from one system in order to purposefully change another system, thus to transfer energy in organized form (as work, thus the ultimate energy quality). No wonder that energy is defined as ability to perform work, and a special quantity exergy is defined as the maximum possible work that may be obtained from a system by bringing it to the equilibrium in a process with reference surroundings (called dead state). The maximum possible work will be obtained if we prevent energy disorganization, thus with limiting reversible processes where the existing non-equilibrium is conserved within interacting systems. Since the energy is conserved during any process, only in ideal reversible processes entropy (measure of energy disorganization or degradation) and exergy (maximum possible work with regard to the reference surroundings) will be conserved, while in real irreversible processes, the entropy will be generated and exergy will be partly (or even fully) destroyed. Therefore, heat transfer and thermal energy are universal manifestations of all natural and artificial (man-made) processes, where all organized potential and/ or quasi-kinetic energies are disorganized or dissipated in the form of thermal energy, in irreversible and spontaneous processes.
Reversibility and Irreversibility: Energy Transfer and Disorganization, and Entropy Generation
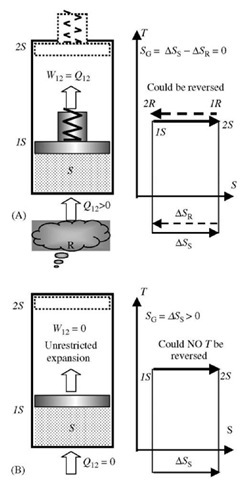
Energy transfer (when energy moves from one system or subsystem to another) through a system boundary and in time, is of kinetic nature, and may be directionally organized as work or directionally chaotic and disorganized as heat. However, the net-energy transfer is in one direction only, from higher to lower energy-potential, and the process cannot be reversed. Thus all processes are irreversible in the direction of decreasing energy-potential (like pressure and temperature) and increasing energy-displacement (like volume and entropy) as a consequence of energy and mass conservation in the universe. This implies that the universe (as isolated and unrestricted system) is expending in space with entropy generation (or increase) as a measure of continuous energy degradation, i.e., energy redistribution and disorganization. It is possible in the limit to have an energy transfer process with infinitesimal potential difference (still from higher to infinitesimally lower potential, P). Then, if infinitesimal change of potential difference direction is reversed (P + dP —*P — dP, with infinitesimally small external energy, since dP —* 0), the process will be reversed too, which is characterized with infinitesimal entropy generation, thus in the limit, without energy degradation (no further energy disorganization) and no entropy gener-ation—thus achieving a limiting, ideal reversible process. Such processes at infinitesimal potential differences, called quasiequilibrium processes, maintain the system equilibrium at any instant but with incremental changes in time. Only quasiequilibrium processes are reversible and vice versa. In effect, the quasiequilibrium reversible processes are infinitely slow processes at infinitesimally small potential differences, but they could be reversed to any previous state, and forwarded to any future equilibrium state, without any “permanent” change to the rest of the surroundings. Therefore, the changes are “fully” reversible, and along with their rate of change and time, completely irrelevant, as if nothing is effectively chan-ging—the time is irrelevant as if it does not exist since it could be reversed (no permanent change and relativity of time). Since the real time cannot be reversed, it is a measure of permanent changes, i.e., irreversibility, which is in turn measured by entropy generation. In this regard the time and irreversible entropy generation are related.[2] Entropy is also a system property, which together with energy defines its equilibrium state, and actually represents the system energy-displacement or random energy disorganization (dissipation) per absolute temperature level over its mass and space it occupies. Therefore, the entropy as property of a system, for a given system state, is the same regardless whether it is reached by reversible heat transfer, Eq. 16, or irreversible heat or irreversible work transfer (adiabatic or caloric processes on Fig. 8B and C). For example, an ideal gas system entropy increase will be the same during a reversible isothermal heat transfer and reversible expansion to a lower pressure (heat-in equal to expansion work-out), as during an irreversible adiabatic unrestricted expansion (no heat transfer and no expansion work) to the same pressure and volume, as illustrated in Fig. 10A and B, respectively.
If heat or work at higher potential (temperature or pressure) than necessary, is transferred to a system, the energy at excess potential will dissipate spontaneously to a lower potential (if left alone) before a new equilibrium state is reached, with entropy generation, i.e., increase of entropy (energy degradation per absolute temperature level). A system will “accept” energy if it is transferred at minimum necessary (infinitesimally higher) or higher potential with regard to the system energy-potential. Furthermore, the higher potential energy will dissipate and entropy increase will be the same as with minimum necessary potential, like in reversible heating process, i.e.:
Fig. 10 (A) Isothermal reversible heat transfer and restricted reversible expansion; (B) Adiabatic unrestricted irreversible expansion of the same initial system to the same final state (Thus the same system entropy change).
However, the source entropy will decrease to a smaller extent over higher potential, thus resulting in overall entropy generation for the two (or all) interacting systems, which may be considered as a combined isolated system (no energy exchange with the rest of the surroundings). The same is true for energy exchange between different system parts (could be considered as subsystems) at different energy potentials (non uniform, not at equilibrium at a given time). Energy at higher potential (say close to boundary within a system) will dissipate (“mix”) to parts at lower energy potential with larger entropy increase than decrease at higher potential, resulting in internal irreversibility and entropy generation, i.e., energy “expansion” over more mass and/or space with lower potential. Entropy is not displacement for heat only as often stated, but also displacement for any energy dissipation (energy disorganization) and the measure of irreversibility. Examples are unrestricted or throttling expansion with no heat exchange but entropy generation. Therefore, entropy generation is fundamental measure of irreversibility or “permanent change.”
Even though directionally organized energy transfer as work, does not possess or generate any entropy (no energy disorganization, Fig. 1B), it is possible to obtain work from the equal amount of disorganized thermal energy or heat if such process is reversible. There are two typical reversible processes where disorganized heat or thermal energy could be entirely transferred into organized work, and vice versa. Namely, they are[2]:
1. reversible expansion at constant thermal energy, e.g., isothermal ideal-gas expansion (8W = 8Q), Fig. 10A, and
2. reversible adiabatic expansion (8W = — dU).
During a reversible isothermal heat transfer and expansion of an ideal-gas system (S), for example, Fig. 10A, the heat transferred from a thermal reservoir (R) will reduce its entropy for (ASR magnitude), wile ideal gas expansion in space (larger volume and lower pressure) will further disorganize its internal thermal energy and increase the gas entropy for (ASs), while in the process an organized expansion work, equivalent to the heat transferred, will be obtained (W12 = Q12). The process could be reversed, and thus it is reversible process with zero total entropy generation (SG = ASs — ASR = 0). On the other hand, if the same initial system (ideal gas) is expanded without any restriction (Fig. 10B, thus zero expansion work) to the same final state, but without heat transfer, the system internal energy will remain the same but more disorganized over the larger volume, resulting in the same entropy increase as during the reversible isothermal heating and restricted expansion. However, this process can not be reversed, since no work was obtained to compress back the system, and indeed the system entropy increase represents the total entropy generation ( Sg = A Ss > 0). Similarly, during reversible adiabatic expansion, the system internal thermal energy will be reduced and transferred in organized expansion work with no change of system entropy (isentropic process), since the reduction of disorganized internal energy and potential reduction of entropy will be compensated with equal increase of disorder and entropy in expanded volume. The process could be reversed back-and-forth (like elastic compression-expansion oscillations of a system) without energy degradation and entropy change, thus an isentropic processes. In reversible processes energy is exchanged at minimum-needed, not higher than needed potential, and isolated systems do not undergo any energy-potential related degradation/disorganization, and with total conservation of entropy. The total non-equilibrium is conserved by reversible energy transfer within interacting systems, i.e., during reversible processes.
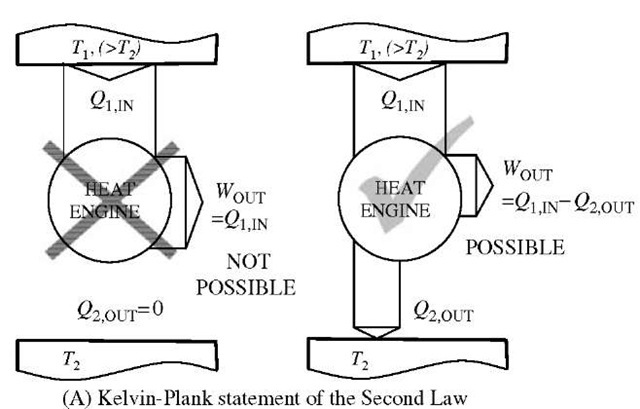
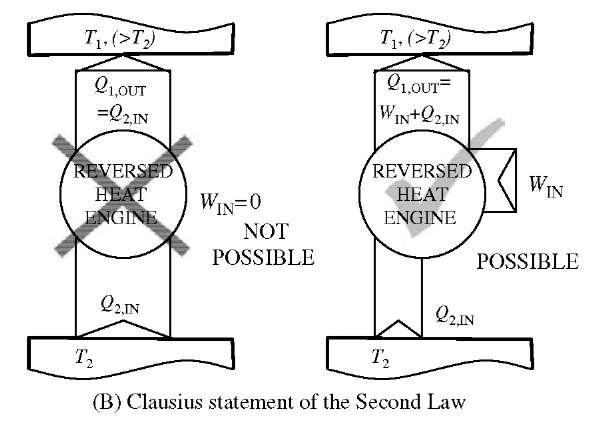
There are two classical statements of the Second Law (both negative, about impossibility), see Fig. 11.[7,8] One is the Kelvin-Plank statement which expresses the never violated fact that it is impossible to obtain work in a continuous cyclic process {perpetual mobile) from a single thermal reservoir (100% efficiency impossible, since it is not possible to spontaneously create non-equilibrium
Fig. 11 The Second Law: (A) Kelvin-Plank, and (B) Clausius statements.
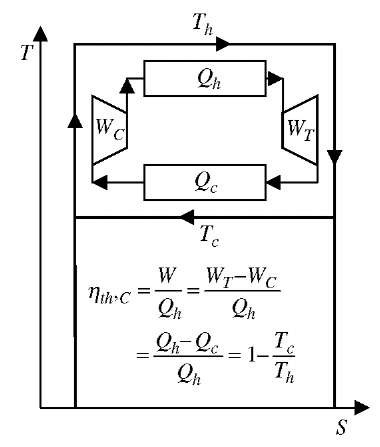
within the single reservoir equilibrium), and the second Clausius statement refers to the direction of heat transfer, expressing the never violated fact that it is impossible for heat transfer to take place by itself (without any work input) from a lower to higher temperature thermal reservoir (it is impossible to spontaneously create non-equilibrium). Actually the two statements imply each other and thus are the same, as well as they imply that all reversible cycles between the two temperature reservoirs (or all reversible processes between the two states) are the most and equally efficient with regards to extracting the maximum work out of a system, or requiring the minimum possible work into the system (thus conserving the existing non-equilibrium). A heat-engine cycle, energy conversion efficiency is defined as:
The reversible cycle efficiency between the two thermal reservoirs does not depend on the cycling system, but only on the ratio of the absolute temperatures of the two reservoirs (T = Tjn and Tml= Tout), known as Carnot cycle efficiency (last part in the Eq. 17, see also below). The latter defines the thermodynamic temperature with the following correlation:
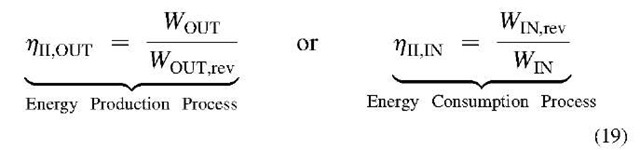
The Second Law efficiency is defined by comparing the real irreversible processes or cycles with the corresponding ideal reversible processes or cycles:
The irreversibility (I) is due to the entropy generation (Sgen) and represents the lost work potential or lost exergy (EX,ioss) with regard to reference system (surroundings at T0 absolute temperature), as expressed by the following correlation:
The irreversibility (I) is due to the entropy generation (Sgen) and represents the lost work potential or lost exergy (EX,ioss) with regard to reference system (surroundings at T0 absolute temperature), as expressed by the following correlation:
Fig. 12 Heat engine ideal Carnot cycle.
Heat Engines
Heat Engines are devices undergoing thermo-mechan-ical cycles (transforming thermal into mechanical energy), similar to one on Fig. 12, with mechanical expansion and compression net-work (W = Qh — Qc), obtained as the difference between the heat transferred to the engine from high temperature heat reservoir (at Th) and rejected to a low (cold) temperature heat reservoir (at Tc), thus converting part of the thermal energy into mechanical work. In a close-system cycle the net-work-out is due to net-work of thermal-expansion and thermal-compression. Therefore, heat engine cycle cannot be accomplished without two thermal reservoirs at different temperatures, one at higher temperature to accomplish thermal expansion and work out, and another at lower temperature to accomplish thermal compression to initial volume and thus complete the cycle. The combustion process itself is an irreversible one, where chemical energy (electrochemical energy binding atoms in reactants’ molecules) is chaotically released during combustion, i.e., converted into random thermal energy of products’ molecules, and cannot be fully converted into directional work energy. The Second Law of Thermodynamics limits the maximum amount of work that could be obtained from thermal energy between any
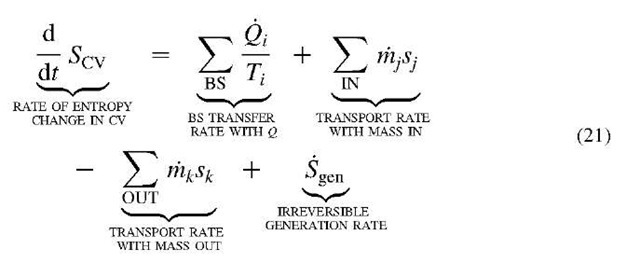
The entropy balance equation for the control-volume flow process, complementing the related energy balance Eq. 11, see Fig. 9, is[5-7]:
two thermal reservoirs at different temperatures, hot Th, and cold Tc, by using the ideal, reversible Carnot cycle, see Fig. 12, with thermal efficiency given by Eq. 22. As an example, consider Tc = 293 K and Th = 2273 K:
two thermal reservoirs at different temperatures, hot Th, and cold Tc, by using the ideal, reversible Carnot cycle, see Fig. 12, with thermal efficiency given by Eq. 22. As an example, consider Tc = 293 K and Th = 2273 K:
where, W = WT — Wc, is the net-work of expansion, usually turbine (WT), and compression (Wc). The maximum efficiency is achieved if heat is supplied at the highest possible temperature Th, and released at the lowest possible temperature Tc. However, both temperatures are limited by the fact that fuel combustion is performed using oxygen with ambient air, resulting in maximum so called adiabatic, stoichiometric combustion temperature Tad, which is for most fuels about 2000°C, or Tad = 2273 K. A part of the heat supplied at hot temperature Th, must be released to the surroundings at cold temperature about Tc = 20°C = 293 K, which results in a Carnot efficiency of 87.1%, see Eq. 22 and Fig. 12. However, the fuel heating value energy QHV = Qad_var , is not all available at the adiabatic temperature of the products, but is distributed over their variable temperature range from initial surrounding temperature before combustion Tc, to final adiabatic temperature Tad. For such a variable heat reservoir, a large number (infinite in the limit) of ideal Carnot engines operating at different temperatures (with dW = dQ), must be employed to achieve a reversible cycle, resulting in the variable-temperature Carnot cycle with the maximum possible combustion-products-to-work conversion efficiency[1]:
The above Eq. 23 is valid if the cyclic medium has constant specific heat, otherwise integration will be required. Due to engine material property limitations and other unavoidable irreversibilities, it is impossible to reach the ideal Carnot efficiency. Different actual heat engines undergo similar but different cycles, depending on the system design. For example, internal combustion engines undergo the Otto cycle with gasoline fuel and the Diesel cycle with diesel fuel, while the steam and gas turbine power plants undergo the Rankine and Brayton cycles, respectively. However, with improvements in material properties, effective component cooling, and combining gas and steam turbine systems, the efficiencies above 50% are being achieved, which is a substantial improvement over the usual 30%-35% efficiency. The ideal Carnot cycle is an important reference to guide researchers and engineers to better understand limits and possibilities for new concepts and performance improvements of real heat engines.
CONCLUDING REMARKS: ENERGY PROVIDES EXISTENCE AND IS CAUSE FOR CHANGE
Energy is fundamental property of a physical system and refers to its potential to maintain a system identity or structure and to influence changes with other systems (via forced interaction) by imparting work (forced directional displacement) or heat (forced chaotic displacement/motion of a system molecular or related structures). Energy exists in many forms: electromagnetic (including light), electrical, magnetic, nuclear, chemical, thermal, and mechanical (including kinetic, elastic, gravitational, and sound); where, for example, electro-mechanical energy may be kinetic or potential, while thermal energy represents overall potential and chaotic motion energy of molecules and/or related micro structure.
The philosophical and practical aspects of energy and entropy, including reversibility and irreversibility could be summarized as follows:
• Energy is a fundamental concept indivisible from matter and space, and energy exchanges or transfers are associated with all processes (or changes), thus indivisible from time.
• Energy is “the building block” and fundamental property of matter and space, and thus a fundamental property of existence. For a given matter (system structure) and space (volume) energy defines the system equilibrium state, and vice versa.
• For a given system state (structure and phase) addition of energy will spontaneously tend to randomly redistribute (disorganize, degrade) over the system smaller microstructure and space it occupies, called thermal energy, equalizing the thermal energy-potential (temperature) and increasing the energy-displacement (entropy), and vice versa.
• Entropy may be transferred from system to system by reversible heat transfer and also generated due to irreversibility of heat and work transfer.
• Energy and mass are conserved within interacting systems (all of which may be considered as a combined isolated system not interacting with its surrounding systems), and energy transfer (in time) is irreversible (in one direction) from higher to lower energy-potential only, which then results in continuous generation (increase) of energy-displacement, called entropy generation, which is fundamental measure of irreversibility, or permanent changes.
• Reversible energy transfer is only possible as a limiting case of irreversible energy transfer at infinitesimally small energy-potential differences, thus in quasiequili-brium processes, with conservation of entropy. Since such changes are reversible, they are not permanent and, along with time, irrelevant.
In summary, energy is providing existence, and if exchanged, it has ability to perform change.
Glossary
Energy: It is fundamental property of a physical system and refers to its potential to maintain a system identity or structure and to influence changes with other systems (via forced-displacement interactions) by imparting work (forced directional displacement) or heat (forced chaotic displacement/motion of a system molecular or related structures). Energy exists in many forms: electromagnetic (including light), electrical, magnetic, nuclear, chemical, thermal, and mechanical (including kinetic, elastic, gravitational, and sound).
Energy Conservation: It may refer to the fundamental law of nature that energy and mass are conserved, i.e., cannot be created or destroyed but only transferred from one form or one system to another. Another meaning of energy conservation is improvement of efficiency of energy processes so that they could be accomplished with minimal use of energy sources and minimal impact on the environment.
Energy Conversion: A process of transformation of one form of energy to another, like conversion of chemical to thermal energy during combustion of fuels, or thermal to mechanical energy using the heat engines, etc.
Energy Efficiency: Ratio between useful (or minimally necessary) energy to complete a process and actual energy used to accomplish that process. Efficiency may also be defined as the ratio between energy used in an ideal energy-consuming process vs energy used in the corresponding real process, or vice versa for an energy-producing process. Energy, as per the conservation law, cannot be lost (destroyed), but part of energy input which is not converted into useful energy is customarily referred to as energy loss.
Entropy: It is an integral measure of thermal energy redistribution (due to heat transfer or irreversible heat generation) within a system mass and/or space (during system expansion), per absolute temperature level. Entropy is increasing from orderly crystalline structure at zero absolute temperature (zero reference) during reversible heating and entropy generation during irreversible energy conversion, i.e., energy degradation or random equi-partition within system material structure and space.
Exergy: It is the maximum system work potential if it is reversibly brought to the equilibrium with reference surroundings, i.e., exergy is a measure of a system non-equilibrium with regard to the reference system.
Heat: It is inevitable (spontaneous) energy transfer due to temperature differences (from higher to lower level), to a larger or smaller degree without control (dissipative) via chaotic (in all directions, non purposeful) displacement/ motion of system molecules and related microstructure, as opposed to controlled (purposeful and directional) energy transfer referred to as work (see below).
Heat Engine: It is a device undergoing thermo-mechanical cycle that partially converts thermal energy into mechanical work and is limited by the Carnot cycle efficiency. The cycle mechanical expansion and compression net-work is obtained due to difference between heat transferred to the engine from a high temperature heat reservoir and rejected to a low temperature reservoir, thus converting part of thermal energy into mechanical work.
Mechanical Energy: It is defined as the energy associated with ordered motion of moving objects at large scale (kinetic) and ordered elastic potential energy within the material structure (potential elastic), as well as potential energy in gravitational field (potential gravitational).
Power: It is the energy rate per unit of time and is related to work or heat transfer processes (different work power or heating power).
System: (also Particle or Body or Object) refers to any, arbitrary chosen but fixed physical or material system in space (from a single particle to system of particles), which is subject to observation and analysis. System occupies so called system volume within its own enclosure interface or system boundary, and thus separates itself from its surroundings, i.e., other surrounding systems.
Temperature: It is a measure of the average quasi-translational kinetic energy associated with the disordered microscopic motion of atoms and molecules relevant for inter-particulate collision and heat transfer, thus temperature is related to relevant particle kinetic energy and not to the particle density in space.
Thermal Energy: It is defined as the energy associated with the random, disordered motion of molecules and potential energy due to intermolecular forces (also associated with phase change), as opposed to the macroscopic ordered energy associated with moving objects at large scale.
Total Internal Energy: It is defined as the energy associated with the random, disordered motion of molecules and intermolecular potential energy (thermal), “binding” potential energy associated with chemical molecular structure (chemical) and atomic nuclear structure (nuclear), as well as with other structural potentials in force fields (electrical, magnetic, etc.). It refers to the “invisible” microscopic energy on the subatomic, atomic and molecular scale.
Work: It is a type of controlled energy transfer when one system is exerting force in a specific direction and thus making a purposeful change (forced displacement) of the other systems. It is inevitably (spontaneously) accompanied, to a larger or smaller degree (negligible in ideal processes), with dissipative (without control) energy transfer referred to as heat (see above).