Concept
The elements are at the heart of chemistry, and indeed they are at the heart of life as well. Every physical substance encountered in daily life is either an element, or more likely a compound containing more than one element. There are millions of compounds, but only about 100 elements, of which only 88 occur naturally on Earth. How can such vast complexity be created from such a small amount of elements? Consider the English alphabet, with just 26 letters, with which an almost infinite number of things can be said or written. Even more is possible with the elements, which greatly outnumber the letters of the alphabet. Despite the relatively great quantity of elements, however, just two make up almost the entire mass of the universe—and these two are far from abundant on Earth. A very small number of elements, in fact, are essential to life on this planet, and to the existence of human beings.
How it works
The Focal Point of Chemistry
Like physics, chemistry is concerned with basic, underlying processes that explain how the universe works. Indeed, these two sciences, along with astronomy and a few specialized fields, are the only ones that address phenomena both on the Earth and in the universe as a whole. By contrast, unless or until life on another planet is discovered, biology has little concern with existence beyond Earth’s atmosphere, except inasmuch as the processes and properties in outer space affect astronauts.
While physics and chemistry address many of the same fundamental issues, they do so in very different ways. To make a gross generalization—subject to numerous exceptions, but nonetheless useful in clarifying the basic difference between the two sciences—physicists are concerned with external phenomena, and chemists with internal ones.
For instance, when physicists and chemists study the interactions between atoms, unless physicists focus on some specialized area of atomic research, they tend to treat all atoms as more or less the same. Chemists, on the other hand, can never treat atoms as though they are just undifferentiated particles colliding in space. The difference in structure between one kind of atom and another, in fact, is the starting-point of chemical study.
Structure of the Atom
An atom is the fundamental particle in a chemical element, or a substance that cannot be broken down into another substance by chemical means. Clustered at the center, or nucleus, of the atom are protons, which have a positive electric charge, and neutrons, which possess no charge.
Spinning around the nucleus are electrons, which are negatively charged. The vast majority of the atom’s mass is made up by the protons and neutrons, which have approximately the same mass, whereas that of the electron is much smaller. The mass of an electron is about 1/1836 that of proton, and 1/1839 that of a neutron.
It should be noted that the nucleus, though it constitutes most of the atom’s mass, is only a tiny portion of the atom’s volume. If the nucleus were the size of a grape, in fact, the electrons would, on average, be located about a mile away.
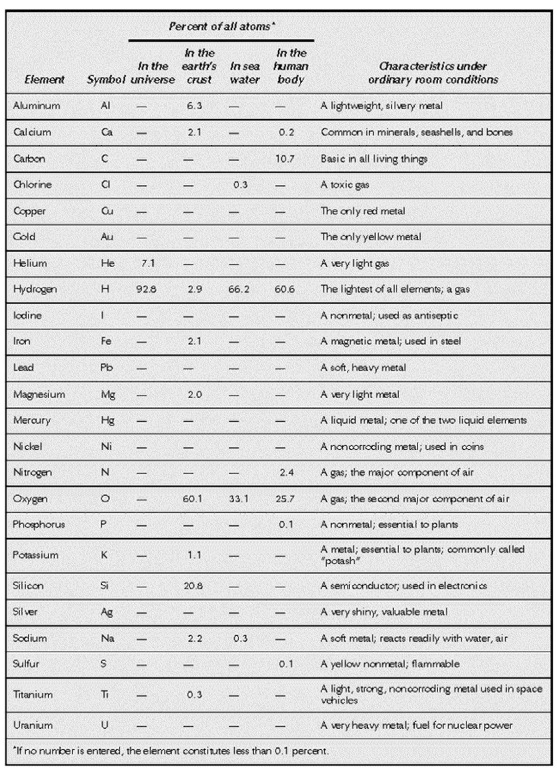
The most common and/or important chemical elements.
Isotopes
Atoms of the same element always have the same number of protons, and since this figure is unique for a given element, each element is assigned an atomic number equal to the number of protons in its nucleus. Two atoms may have the same number of protons, and thus be of the same element, yet differ in their number of neutrons. Such atoms are called isotopes.
Isotopes are generally represented as follows: “a”S, where S is the symbol of the element, a is the atomic number, and m is the mass number—the sum of protons and neutrons in the atom’s nucleus. For the stable silver isotope designated as 93 i7Ag, for instance, Ag is the element symbol (discussed below); 47 its atomic number; and 93 the mass number. From this, it is easy to discern that this particular stable isotope has 46 neutrons in its nucleus.
Because the atomic number of any element is established, sometimes isotopes are represented simply with the mass number thus: 93Ag. They may also be designated with a subscript notation indicating the number of neutrons, so that one can obtain this information at a glance without having to do the arithmetic. For the silver isotope 39/33 shown here, this is written as 333Ag46. Isotopes are sometimes indicated by simple nomenclature as well: for instance, carbon-12 or carbon-13.
Ions
The number of electrons in an atom is usually the same as the number of protons, and thus most atoms have a neutral charge. In certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. The electrons are not “lost” when an atom becomes an ion: they simply go elsewhere.
Aluminum (Al), for instance, has an atomic number of 13, which tells us that an aluminum atom will have 13 protons. Given the fact that every proton has a positive charge, and that most atoms tend to be neutral in charge, this means that there are usually 13 electrons, with a negative charge, present in an atom of aluminum. Aluminum may, however, form an ion by losing three electrons.
Cations
After its three electrons have departed, the remaining aluminum ion has a net positive charge of 3, represented as +3. How do we know this? Initially the atom had a charge of +13 + (-13) = 0. With the exit of the 3 electrons, leaving behind only 10, the picture changes: now the charge is +13 + (-10) = +3.
When a neutral atom loses one or more electrons, the result is a positively charged ion, or cation (pronounced KAT-ie-un). Cations are represented by a superscript number and plus sign after the element symbol: Al3+, for instance,represents the aluminum cation described above. (Some chemists represent this with the plus sign before the number—for example, Al+3.) A cation is named after the element of which it is an ion: thus the ion we have described is called either the aluminum ion, or the aluminum cation.
Anions
When a neutrally charged atom gains electrons, and as a result acquires a negative charge, this type of ion is known as an anion (AN-ie-un). Anions can be represented symbolically in much the same way of cations: C1-, for instance, is an anion of chlorine that forms when it acquires an electron, thus assuming a net charge of -1. Note that the 1 is not represented in the superscript notation, much as people do not write 101. In both cases, the 1 is assumed, whereas any number higher than 1 is shown.
The anion described here is never called a chlorine anion; rather, anions have a special nomenclature. If the anion represents, as is the case here, a single element, it is named by adding the suffix -ide to the name of the original element name: hence it would be called chloride. Other anions involve more than one element, and in these cases other rules apply for designating names. A few two-element anions use the -ide ending; such is the case, for instance, with a deadly mixture of carbon and nitrogen (CN-), better known as cyanide.
For anions involving oxygen, there may be different prefixes and suffixes, depending on the relative number of oxygen atoms in the anion.
Real-Life Applications
The Periodic Table
Note that an ion is never formed by a change in the number of protons: that number, as noted earlier, is a defining characteristic of an element. If all we know about a particular atom is that it has one proton, we can be certain that it is an atom of hydrogen. Likewise, if an atom has 79 protons, it is gold.
Knowing these quantities is not a matter of memorization: rather, one can learn this and much more by consulting the periodic table of elements. The periodic table is a chart, present in virtually every chemistry classroom in the world,showing the elements arranged in order of atomic number. Elements are represented by boxes containing the atomic number, element symbol, and average atomic mass, in atomic mass units, for that particular element. Vertical columns within the periodic table indicate groups or “families” of elements with similar chemical characteristics.
These groups include alkali metals, alkaline earth metals, halogens, and noble gases. In the middle of the periodic table is a wide range of vertical columns representing the transition metals, and at the bottom of the table, separated from it, are two other rows for the lanthanides and actinides.
An Overview of the Elements
As of2001, there 112 elements, of which 88 occur naturally on Earth. (Some sources show 92 naturally occurring elements; however, a few of the elements with atomic numbers below 92 have not actually been found in nature.) The others were created synthetically, usually in a laboratory, and because these are highly radioactive, they exist only for fractions of a second. The number of elements thus continues to grow, but these “new” elements have little to do with the daily lives of ordinary people. Indeed, this is true even for some of the naturally occurring elements: few people who are not chemically trained, for instance, are able to identify thulium, which has an atomic number of 69.
Though an element can theoretically exist as a gas, liquid, or a solid, in fact the vast majority of elements are solids. Only 11 elements—the six noble gases, along with hydrogen, nitrogen, oxygen, fluorine, and chlorine—exist in the gaseous state at a normal temperature of about 77°F (25°C). Just two are liquids at normal temperature: mercury, a metal, and the non-metal bromine. (The metal gallium becomes liquid at just 85.6°F, or 29.76°C.) The rest are all solids.
The noble gases are monatomic, meaning that they exist purely as single atoms. So too are the “noble metals,” such as gold, silver, and platinum. “Noble” in this context means “set apart”: noble gases and noble metals are known for their tendency not to react to, and hence not to bond with, other elements. On the other hand, a number of other elements are described as diatomic, meaning that two atoms join to form a molecule.
Names of the Elements
Element Symbol
As noted above, the periodic table includes the element symbol or chemical symbol—a one-or two-letter abbreviation for the name of the element. Many of these are simple one-letter designations: O for oxygen, or C for carbon. Others are two-letter abbreviations, such as Ne for neon or Si for silicon. Note that the first letter is always capitalized, and the second is always lowercase.
In many cases, the two-letter symbols indicate the first and second letters of the element’s name, but this is far from universal. Cadmium, for example, is abbreviated Cd, while platinum is Pt. In other cases, the symbol seems to have nothing to do with the element name as it is normally used: for instance, Au for gold or Fe for iron.
Many of the one-letter symbols indicate elements discovered early in history. For instance, carbon is represented by C, and later “C” elements took two-letter designations: Ce for cerium, Cr for chromium, and so on. But many of those elements with apparently strange symbols were among the first discovered, and this is precisely why the symbols make little sense to a person who does not recognize the historical origins of the name.
Historical background on some element names
For many years, Latin was the language of communication between scientists from different nations; hence the use of Latin names such as aurum (“shining dawn”) for gold, or ferrum, the Latin word for iron. Likewise, lead (Pb) and sodium (Na) are designated by their Latin names, plumbum and natrium, respectively.
Some chemical elements are named for Greek or German words describing properties of the element—for example, bromine (Br), which comes from a Greek word meaning “stench.” The name of cobalt comes from a German term meaning “underground gnome,” because miners considered the metal a troublemaker. The names of several elements with high atomic numbers reflect the places where they were originally discovered or created: francium, germanium, americium, californium.
Americium and californium, with atomic numbers of 95 and 98 respectively, are among those elements that do not occur naturally, but were created artificially. The same is true of several elements named after scientists—among them einsteinium, after Albert Einstein (18791955), and nobelium after Alfred Nobel (18331896), the Swedish inventor of dynamite who established the Nobel Prize.
Abundance of Elements
In the universe
The first two elements on the periodic table, hydrogen and helium, represent 99.9% of the matter in the entire universe. This may seem astounding, but Earth is a tiny dot within the vastness of space, and hydrogen and helium are the principal elements in stars.
All elements, except for those created artificially, exist both on Earth and throughout the universe. Yet the distribution of elements on Earth is very, very different from that in other places—as well it should be, given the fact that Earth is the only planet known to support life. Hydrogen, for instance, constitutes only about 0.87%, by mass, of the elements found in the planet’s crust, waters, and atmosphere. As for helium, it is not even among the 18 most abundant elements on Earth.
On earth
That great element essential to animal life, oxygen, is by far the most plentiful on Earth, representing nearly half—49.2%—of the total mass of atoms found on this planet. (Here the term “mass” refers to the known elemental mass of the planet’s atmosphere, waters, and crust; below the crust, scientists can only speculate, though it is likely that much of Earth’s interior consists of iron.) Together with silicon (25.7%), oxygen accounts for almost exactly three-quarters of the elemental mass of Earth. Add in aluminum (7.5%), iron (4.71%), calcium (3.39%), sodium (2.63%), potassium (2.4%),and magnesium (1.93%), and these eight elements make up about 97.46% of Earth’s material.
In addition to hydrogen, whose distribution is given above, nine other elements account for a total of 2% of Earth’s composition: titanium (0.58%), chlorine (0.19%), phosphorus (0.11%), manganese (0.09%), carbon (0.08%), sulfur (0.06%), barium (0.04%), nitrogen (0.03%), and fluorine (0.03%). The remaining 0.49% is made up of various elements.
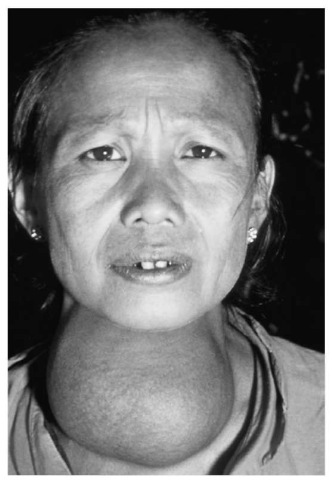
This woman’s goiter is probably the result of a lack of iodine in her diet.
Elements In the Human Body
Fans of science-fiction are familiar with the phrase “carbon-based life form,” which is used, for instance, by aliens in sci-fi movies to describe humans. In fact, the term is a virtual redundancy on Earth, since all living things contain carbon.
Essential though it is to life, carbon as a component of the human body takes second place to oxygen, which is an even larger proportion of the body’s mass—65.0%—than it is of the Earth. Carbon accounts for 18%, and hydrogen for 10%, meaning that these three elements make up 93% of the body’s mass. Most of the remainder is taken up by 10 other elements: nitrogen (3%), calcium (1.4%), phosphorus (1.0%), magnesium (0.50%), potassium (0.34%), sulfur (0.26%), sodium (0.14%), chlorine (0.14%), iron (0.004%), and zinc (0.003%).
Trace elements
As small as the amount of zinc is in the human body, there are still other elements found in even smaller quantities. These are known as trace elements, because only traces of them are present in the body. Yet they are essential to human well-being: without enough iodine, for instance, a person can develop a goiter, a large swelling in the neck area. Chromium helps the body metabolize sugars, which is why people concerned with losing weight and/or toning their bodies through exercise may take a chromium supplement.
Key Terms
Anion: The negative ion that results when an atom gains one or more electrons. An anion (pronounced “AN-ie-un”) of an element is never called, for instance, the chlorine anion. Rather, for an anion involving a single element, it is named by adding the suffix -ide to the name of the original element—hence, “chloride.” Other rules apply for more complex anions. Atom: The smallest particle of an element. An atom can exist either alone or in combination with other atoms in a molecule. Atoms are made up of protons, neutrons, and electrons. An atom that loses or gains one or more electrons, and thus has a net charge, is an ion. Atoms that have the same number of protons—that is, are of the same element—but differ in number of neutrons are known as isotopes. atomic mass unit: An SI unit (abbreviated amu), equal to 1.66 • 10-24 g, for measuring the mass of atoms. atomic number: The number of protons in the nucleus of an atom. Since this number is different for each element, elements are listed on the periodic table in order of atomic number.
Average atomic mass: A figure used by chemists to specify the mass—in atomic mass units—of the average atom in
a large sample. If a substance is a compound, the average atomic mass of all atoms in a molecule of that substance must be added together to yield the average molecular mass of that substance.
Cation: The positive ion that results when an atom loses one or more electrons. A cation (pronounced KAT-ie-un) is named after the element of which it is an ion and thus is called, for instance, the aluminum ion or the aluminum cation. Chemical symbol: Another term for element symbol.
Compound: A substance made up of atoms of more than one element. These atoms are usually joined in molecules. diatomic: A term describing an element that exists as molecules composed of two atoms. This is in contrast to monatom-ic elements.
Electron: Negatively charged particles in an atom. Electrons, which spin around the protons and neutrons that make up the atom’s nucleus, constitute a very small portion of the atom’s mass. The number of electrons and protons is the same, thus canceling out one another. But when an atom loses or gains one or more electrons, however—thus becoming an ion—it acquires a net electric charge.
Element: A substance made up of only one kind of atom. Unlike compounds, elements cannot be chemically broken into other substances.
Element symbol: A one- or two-letter abbreviation for the name of an element. These may be a single capitalized letter (O for oxygen), or a capitalized letter followed by a lowercase one (Ne for neon). Sometimes the second letter is not the second letter of the element name (for example, Pt for platinum). In addition, the symbol may refer to an original Greek or Latin name, rather than the name used now, is the case with Au (aurum) for gold.
Ion: An atom that has lost or gained one or more electrons, and thus has a net electric charge.
Isotopes: Atoms that have an equal number of protons, and hence are of the same element, but differ in their number of neutrons.
Mass number: The sum of protons and neutrons in an atom’s nucleus. Where an isotope is represented, the mass number is placed above the atomic number to the left of the element symbol.
Molecule: A group of atoms, usually but not always representing more than one element, joined in a structure. Compounds are typically made of up molecules.
Monatomic: A term describing an element that exists as single atoms. This in contrast to diatomic elements. neutron: A subatomic particle that has no electric charge. Neutrons are found at the nucleus of an atom, alongside protons.
Nucleus: The center of an atom, a region where protons and neutrons are located, and around which electrons spin.
Periodic table of elements: A chart that shows the elements arranged in order of atomic number, along with element symbol and the average atomic mass (in atomic mass units) for that particular element. Vertical columns within the periodic table indicate groups or “families” of elements with similar chemical characteristics.
Proton: A positively charged particle in an atom. Protons and neutrons, which together form the nucleus around which electrons spin, have approximately the same mass—a mass that is many times greater than that of an electron. The number of protons in the nucleus of an atom is the atomic number of an element.
Even arsenic, lethal in large quantities, is a trace element in the human body, and medicines for treating illnesses such as the infection known as “sleeping sickness” contain tiny amounts of arsenic. Other trace elements include cobalt, copper, fluorine, manganese, molybdenum, nickel, selenium, silicon, and vanadium.
Maintaining and improving health
Though these elements are present in trace quantities within the human body, that does not mean that exposure to large amounts of them is healthy. Arsenic, of course, is a good example; so too is aluminum. Aluminum is present in unexpected places: in baked goods, for instance, where a compound containing aluminum (baking powder) is sometimes used in the leavening process; or even in cheeses, as an aid to melting when heated. The relatively high concentrations of aluminum in these products, as well as fluorine in drinking water, has raised concerns among some scientists.
Generally speaking, an element is healthy for the human body in proportion to its presence in the body. With trace elements and others that are found in smaller quantities, however, it is sometimes possible and even advisable to increase the presence of those elements by taking dietary supplements. Hence a typical multivitamin contains calcium, iron, iodine, magnesium, zinc, selenium, copper, chromium, manganese, molybdenum, boron, and vanadium.
For most of these, recommended daily allowances (RDA) have been established by the federal government. Usually, people do not take sodium as a supplement, though—Americans already get more than their RDA of sodium through salt, which is overly abundant in the American diet.
