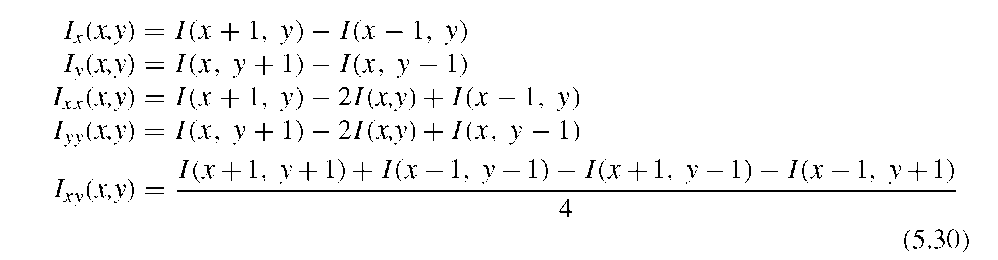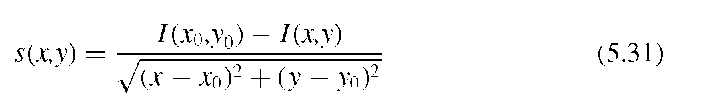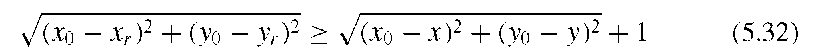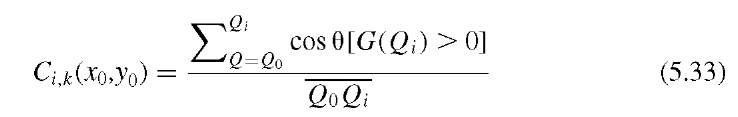Adaptive methods have been under development for many years and are finding their way into biomedical image analysis. In most cases, adaptive filters constitute an intermediate step in the image processing chain. For this reason, adaptive filtering is found most frequently as a tool rather than as the research objective of a study. With the where the partial derivatives may be implemented through central differences as described by
development of computers that have higher processing power, the algorithms available become considerably more complex. An early example of adaptive contrast enhancement is a study on the effect of local histogram equalization in CT images by Pitzer et al.31 The focus of this study was not only the effect of local histogram equalization, but primarily a computationally effective implementation suitable for the processing power available at that time. Another early study demonstrated the superiority of adaptive sharpening over nonadaptive unsharp masking in image enhancement for the detection of lung nodules in chest x-ray images.12 The conventional unsharp masking step made use of two masks, where the image was convolved with box filters of 35 x 35and101 x 101 pixels window size, respectively. The resulting unsharp masks were subtracted from the original image with weight factors of 0.5 and 1.0. This method of unsharp masking is often referred to as the difference-of-Gaussian (DoG) operator and the operation has a strong highpass character. In the adaptive variant, the same convolution was used to obtain unsharp masks, but the weight factors were allowed to vary from 0 to 0.5 and 0 to 1.0, respectively, depending on the radiolucency of the local area. In dark regions of the digitized image (i.e., the radiolucent regions of the lung), the weight factors were low, and consequently, the sharpening effect of the filter was also low. The maximum effect of the filter was reached in the projections of bone and probably in the nodule regions. In an ROC analysis, radiologists most reliably identified the nodules in the adaptively filtered x-ray images, whereas there was no difference in unfiltered and nonadaptively filtered images. The effect of the two filters (nonadaptive unsharp masking and locally adaptive unsharp masking) is demonstrated in Figure 5.14.
FIGURE 5.14 Effect of a locally adaptive unsharp masking filter on a scanned chest x-ray image. The original image (A) shows a high dynamic range with low contrast in critical regions, such as the lung region with the nodule (circled with a marker). Note also the two buttons in the top part of the image. Nonadaptive DoG (B) overly increases local contrast in areas of high radiolucency (i.e., the lung area), while the region of the spinal column retains low contrast. The critical areas in the lung appear overly sharp, even partially burned out. The adaptive filter that reduces its strength in areas of high radiolucency (C) balances contrast and, according to the study, allows radiologists to detect lung nodules more reliably.
Another study where an adaptive filter was contrasted with an equivalent non-adaptive filter was based on MR data that were affected by image bias.14 Image bias is conventionally removed by homomorphic (i.e., multiplicative) highpass filtering. In the new algorithm, a bias field model was generated iteratively by first generating a threshold map, then by creating an unsharp mask of the tissue areas in the MR image, and finally, by dividing the threshold map by the unsharp mask. The process was repeated about five times, after which features such as blood vessels, were more clearly visible than in homomorphic filtered images. Adaptive threshold masking also leads to a more uniform histogram.
X-ray mammography continues to be a challenging area for image analysis, with the goal to aid the radiologist in the detection of microcalcifications or suspicious masses. Adaptive filtering steps are frequently employed. For example, an adaptive filter that emphasizes medium-contrast areas was the basis of density-weighted contrast enhancement segmentation.30 With additional image processing steps, namely, region growing of the objects found in the adaptive segmentation step and subsequent morphological classification, a reliable detection of suspicious masses with 97% true-positive detection and 35.5% false-positives was achieved.
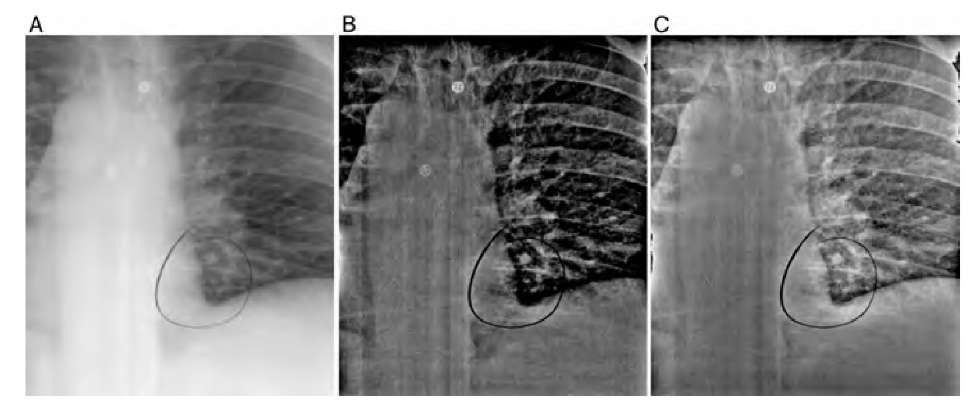
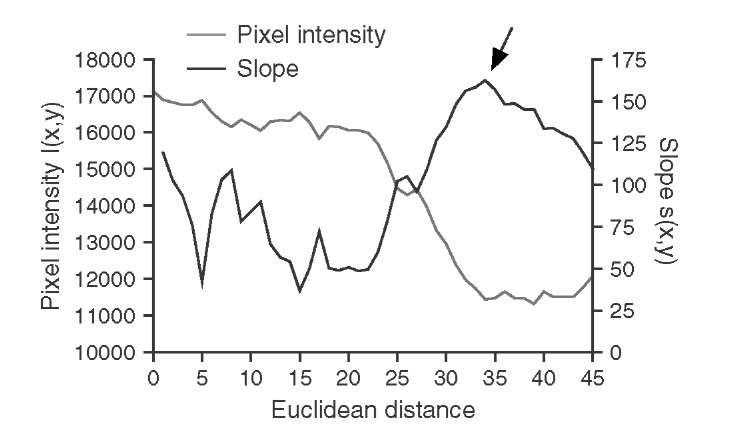
A fundamentally different segmentation approach for the segmentation of micro-calcifications was presented by Bankman et al.5 The hill-climbing algorithm presented in this study is based on the fact that the edge of a microcalcification is a closed contour around an area of higher intensity. This observation implies that a microcalcification has one pixel with a local intensity maximum I(x0,y0), which can be found algo-rithmically. To find the edges of the microcalcifications, pixel intensities are probed along 16 straight lines at 0°, 22.5°, 45°,… with respect to the horizontal, starting at the local intensity maximum at x0,y0. For each line, a slope s(x,y) is defined through
Pixels that are added to the cluster serve iteratively as reference pixels, and the iteration stops when no more pixels are added to the cluster. The result is a
and the edge is defined as the highest slope value s(x,y) along the probing line (Figure 5.15). With this method, 16 edge points can be found. In addition, the edge direction is defined as the line perpendicular to the probing line. The actual hill climbing takes place in the second part of the algorithm: Starting with the 16 edge pixels as reference pixels, each pixel that is 8-connected to a reference pixel is added to the feature cluster (a) if it is an uphill pixel (i.e., its intensity is higher than the intensity of the reference pixel) and if it lies on the local maximum side of a line that goes through the reference pixel and is perpendicular to the line connecting the reference pixel to the local maximum, or (b) if its intensity is lower than the intensity of the reference pixel and the pixel is closer to the local maximum by the distance of one or more pixels, that is,pixel mask that defines the area of microcalcification. Thus, the hill-climbing algorithm segments the microcalcification, but due to the definition of the slope in Equation (5.31), it does so in an adaptive manner: Hill climbing is independent of the local intensity, and it is also independent of local statistics. The hill-climbing algorithm has no external parameters except the maximum length of the probing ray and is therefore excellently suited for fully unsupervised segmentation.
FIGURE 5.15 Intensity and slope along one representative probing line. The probing line starts at the local maximum (Euclidean distance 0), and pixel intensity drops off somewhat monotonically with increasing distance. The slope shows a distinct maximum at the steepest drop-off, approximately 35 pixels from the local maximum (arrow). This point on the probing line is marked as an edge point.
An interesting filter in the context of the detection of suspicious breast masses is the iris filter,19 which is based on the same idea of enhancing image areas to which the gradient field converges: that is, uphill, when the image is interpreted as an elevation landscape. To implement the iris filter, a number of probing lines of equal length R start radially at an arbitrary pixel at x0,y0. For each pixel along these lines, the convergence angle 0 is determined as the angle between the gradient direction and the direction of the line. If the gradient exists (i.e., is nonzero), cos 0 therefore indicates the degree of convergence, because cos 0 is close to unity when the gradient points toward x0,y0 and close to zero when the gradient is perpendicular to the probing line. The degree of convergence for a given point i on probing line k is defined as
where the expression [G(Qi) > 0] assumes the value 1 if a gradient G at pixel Qi exists (i.e., is nonzero) and 0 otherwise. For each probing line, the maximum convergence Ck(x0,y0) is determined as the maximum of all Ci, k of a probing line k. The final output value of the iris filter at x0,y0 is the average of all Ck(x0,y0). A variant of the iris filter was proposed by Varela et al.39 where the convergence determination on each probing ray starts at a minimum distance Rmin from the central point x0,y0 rather than at the center point itself, which reduces noise influence and therefore improves the contrast of suspicious masses. The iris filter was followed by segmentation with a histogram-dependent adaptive threshold and subsequent feature classification. Final evaluation was done using a neural network. The iris filter is a powerful enhancement function for convex shapes with increasing intensity toward the center. However, the iris filter requires an additional segmentation step, as opposed to the hill-climbing algorithm, which implicitly segments the area.
Ultrasound imaging is another modality where adaptive filters have been shown to be a powerful tool in image enhancement. Ultrasound images are generally affected by a random texture of relatively large scale, which has been described as intensity-and location-dependent: The noise level increases with the overall signal intensity, and lateral (but not axial) speckle size increases with depth.26 One example where a locally adaptive minimum mean-square filter as introduced in Section 5.1 was used to reduce variations within adjoining regions (blood region and myocardial region in the heart) was presented by Nillesen et al.25 Noise reduction was performed with an adaptive filter similar to the filter described in Equation (5.3) where the filter strength was controlled locally by the degree of homogeneity, defined as ct(i.e., the intensity variance divided by the mean intensity). Application of this filter strongly reduced the echo level overlap between blood and myocardium, thus improving segmentation results.
A different adaptive filter, adaptive lightening and darkening, was found to be a core step in the segmentation of tissue affected by periventricular leukomalacia in the neonatal brain.35 Once again, ultrasound noise speckles obscured the difference between normal and diseased tissue. Adaptive lightening is defined as increasing the intensity value of a pixel by 1 when the intensity of both pixels of a diagonally opposite pixel pair are higher than the central pixel. In this context, there are four pixel pairs (horizontal, vertical, and two diagonal neighbor pairs), but the horizontal pixel pair is not considered, probably because of the anisotropic nature of the speckles. Conversely, adaptive darkening is defined as decreasing the intensity value by 1 when both pixel intensities of the same pixel pairs are lower than the intensity of the central pixel. Since diseased tissue is brighter (more echogenic) than healthy tissue, brightening is applied to image areas above a certain threshold, and darkening is applied to areas below a different and lower threshold. In the transition zone in between the thresholds, weaker darkening is applied. The result of this filter is a form of texture smoothing, but in a directed manner. Within-region contrast is reduced, while at the same time the overall intensity of diseased tissue is raised and the overall intensity of healthy tissue is lowered. Final segmentation took place by means of active contours.
With the goal of providing the radiologist with a subjectively adjustable speckle suppression filter, Thakur and Anand36 proposed a filter termed the gray-level cooccurrence matrix-based adaptive speckle filter. The idea is based on region growing, where region growth and region smoothing are based on second-order statistical parameters: the local mean intensity ^ and the local intensity variance ct2. The noise variance is assumed to scale with the intensity (i.e., ct2 a This assumption allows use of the ratio ctas a measure of homogeneity. The co-occurrence matrix provides several texture descriptors, such as the local contrast and the inverse difference moment9 (see Section 8.2). The adaptive speckle filter is based on these texture parameters, where, starting from a square window, the region is shrunk and areas of high contrast and low inverse difference moment (i.e., probably containing discontinuities) are excluded from the subsequent region-growing step. In the next step, regions are grown along homogeneous areas with low contrast and high inverse difference moment, and these regions are smoothed using mean-value computation. Depending on various filter settings, the ultrasound image can be freed almost completely of speckle noise while the contrast of critical regions is preserved.
Various other methods of adaptive filtering exist in different contexts. For example, an improved thresholding algorithm for three-dimentional micro-CT images of trabecular bone has been presented.8 In this approach, the core of the segmentation was performed using a three-dimentional Sobel operator on a smoothed version of the original image volume. A hysteresis threshold provided an edge map. A local threshold map was formed to include or exclude specific edge voxels to provide a smooth edge contour. Examples for the application of the anisotropic diffusion filter (Section 5.1) were given in a review article.43 In this study, the superiority of the anisotropic diffusion filter as a processing step in the segmentation of the brain cortex surface in MR images was shown. Finally, active contours ("snakes"; see topic 6) are frequently employed to parametrize the contour of a feature. Even the snake algorithm can be made adaptive by employing a local modified trimmed mean noise-reduction step in the vicinity of a snake vertex. In addition, for the specific application of snakes in ultrasound images, where edges have a relatively low slope, the gradient force usually employed is replaced by a ramp integration force to steer the snake toward the edge.10
Adaptive filters have become part of the fundamental image processing toolbox. A selection of adaptive filtering, image enhancement, and segmentation algorithms was presented in this topic. As computers become more powerful, more complex adaptive image processing algorithms are likely to be developed. In the future, adaptive filtering will probably be combined more and more often with other techniques. Examples include frequency-domain adaptive filters,21 the combination of wavelet decomposition with adaptive thresholding for ultrasound speckle reduction,16 or adaptive wavelet filtering techniques for speckle reduction in optical coherence tomography images.2 Finally, the combination of adaptive filters with artificial intelligence methods is a subject of active research.29 There are three major artificial intelligence techniques that are suitable to improving adaptive filtering: fuzzy logic, neural networks, and evolutionary computation. With fuzzy logic, a more human-vision-based approach to characterizing image areas as dark or bright, as having an edge or being smooth, or to characterize properties of different textures is possible. One recent example is the combination of the adaptive median filter with fuzzy logic to reduce heavy shot noise in MR images.15 Neural networks are frequently employed when a closed-form description of classifiers is not possible. A training image set is used to train the network, and the network is then to some extent capable of applying the learned criteria to new images. With evolutionary computation, optimization problems (such as image enhancement) can be performed using genetic algorithms. One recent example is a genetic algorithm attempt at ultrasound speckle reduction.24 Genetic algorithms are search algorithms with a variable parameter space and a fitness function. The parameter space (the "genetic representation") can be randomly mutated and its effect on the fitness function observed. The simulation of evolutionary processes can be highly detailed with mutation, selection, breeding, crossover inheritance, and other phenomena.