Wegener’s Granulomatosis
Definition
Wegener’s granulomatosis is a distinct clinicopathologic entity characterized by granulomatous vasculitis of the upper and lower respiratory tracts together with glomerulonephritis. In addition, variable degrees of disseminated vasculitis involving both small arteries and veins may occur.
Incidence and Prevalence
Wegener’s granulomatosis is an uncommon disease with an estimated prevalence of 3 per 100,000. It is extremely rare in blacks compared with whites; the male-to-female ratio is 1:1.The disease can be seen at any age; ~15% of patients are <19 years of age, but only rarely does the disease occur before adolescence; the mean age of onset is ~40 years.
Pathology and Pathogenesis
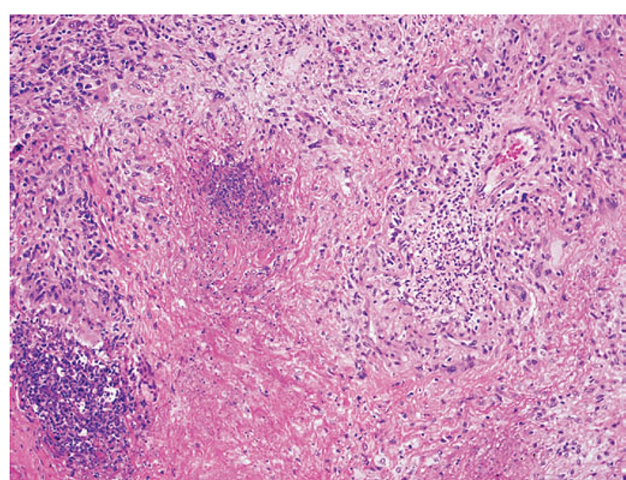
The histopathologic hallmarks of Wegener’s granulomatosis are necrotizing vasculitis of small arteries and veins together with granuloma formation, which may be either intravascular or extravascular (Fig. 10-2).
FIGURE 10-2
Lung histology in Wegener’s granulomatosis. This area of geographic necrosis has a serpiginous border of histiocytes and giant cells surrounding a central necrotic zone. Vasculitis is also present with neutrophils and lymphocytes infiltrating the wall of a small arteriole (upper right).
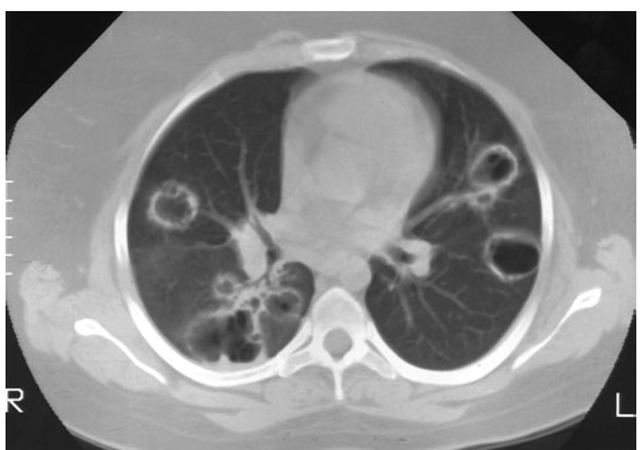
Lung involvement typically appears as multiple, bilateral, nodular cavitary infiltrates (Fig. 10-3), which on biopsy almost invariably reveal the typical necrotizing granulomatous vasculitis. Upper airway lesions, particularly those in the sinuses and nasopharynx, typically reveal inflammation, necrosis, and granuloma formation, with or without vasculitis.
In its earliest form, renal involvement is characterized by a focal and segmental glomerulitis that may evolve into a rapidly progressive crescentic glomerulonephritis. Granuloma formation is only rarely seen on renal biopsy. In contrast to other forms of glomerulonephritis, evidence of immune complex deposition is not found in the renal lesion ofWegener’s granulomatosis. In addition to the classic triad of disease of the upper and lower respiratory tracts and kidney, virtually any organ can be involved with vasculitis, granuloma, or both.
FIGURE 10-3
Computed tomography scan of a patient with Wegener’s granulomatosis. The patient developed multiple, bilateral, and cavitary infiltrates.
The immunopathogenesis of this disease is unclear, although the involvement of upper airways and lungs with granulomatous vasculitis suggests an aberrant cell-mediated immune response to an exogenous or even endogenous antigen that enters through or resides in the upper airway. Chronic nasal carriage of Staphylococcus aureus has been reported to be associated with a higher relapse rate of Wegener’s granulomatosis; however, there is no evidence for a role of this organism in the pathogenesis of the disease.
Peripheral blood mononuclear cells obtained from patients with Wegener’s granulomatosis manifest increased secretion of IFN-γ but not of IL-4, IL-5, or IL-10 compared to normal controls. In addition, TNF-α production from peripheral blood mononuclear cells and CD4+ T cells is elevated. Furthermore, monocytes from patients with Wegener’s granulomatosis produce increased amounts of IL-12. These findings indicate an unbalanced TH1-type T cell cytokine pattern in this disease that may have pathogenic and perhaps ultimately therapeutic implications.
A high percentage of patients with Wegener’s granulomatosis develop ANCA, and these autoantibodies may play a role in the pathogenesis of this disease (see above).
Clinical and Laboratory Manifestations
Involvement of the upper airways occurs in 95% of patients with Wegener’s granulomatosis. Patients often present with severe upper respiratory tract findings such as paranasal sinus pain and drainage and purulent or bloody nasal discharge, with or without nasal mucosal ulceration (Table 10-4). Nasal septal perforation may follow, leading to saddle nose deformity. Serous otitis media may occur as a result of eustachian tube blockage. Subglottic tracheal stenosis resulting from active disease or scarring occurs in ~16% of patients and may result in severe airway obstruction.
Pulmonary involvement may be manifested as asymptomatic infiltrates or may be clinically expressed as cough, hemoptysis, dyspnea, and chest discomfort. It is present in 85-90% of patients. Endobronchial disease, either in its active form or as a result of fibrous scarring, may lead to obstruction with atelectasis.
Eye involvement (52% of patients) may range from a mild conjunctivitis to dacryocystitis, episcleritis, scleritis, granulomatous sclerouveitis, ciliary vessel vasculitis, and retroorbital mass lesions leading to proptosis.
TABLE 10-4
WEGENER’S GRANULOMATOSIS: FREQUENCY OF CLINICAL MANIFESTATIONS IN 158 PATIENTS STUDIED AT THE NATIONAL INSTITUTES OF HEALTH
|
MANIFESTATION |
PERCENT AT DISEASE ONSET |
PERCENT THROUGHOUT COURSE OF DISEASE |
|
Kidney |
||
|
Glomerulonephritis |
18 |
77 |
|
Ear/nose/throat |
73 |
92 |
|
Sinusitis |
51 |
85 |
|
Nasal disease |
36 |
68 |
|
Otitis media |
25 |
44 |
|
Hearing loss |
14 |
42 |
|
Subglottic stenosis |
1 |
16 |
|
Ear pain |
9 |
14 |
|
Oral lesions |
3 |
10 |
|
Lung |
45 |
85 |
|
Pulmonary infiltrates |
25 |
66 |
|
Pulmonary nodules |
24 |
58 |
|
Hemoptysis |
12 |
30 |
|
Pleuritis |
10 |
28 |
|
Eyes |
||
|
Conjunctivitis |
5 |
18 |
|
Dacryocystitis |
1 |
18 |
|
Scleritis |
6 |
16 |
|
Proptosis |
2 |
15 |
|
Eye pain |
3 |
11 |
|
Visual loss |
0 |
8 |
|
Retinal lesions |
0 |
4 |
|
Corneal lesions |
0 |
1 |
|
Iritis |
0 |
2 |
|
Othera |
||
|
Arthralgias/arthritis |
32 |
67 |
|
Fever |
23 |
50 |
|
Cough |
19 |
46 |
|
Skin abnormalities |
13 |
46 |
|
Weight loss (>10% body weight) |
15 |
35 |
|
Peripheral neuropathy |
1 |
15 |
|
Central nervous system disease |
1 |
8 |
|
Pericarditis |
2 |
6 |
|
Hyperthyroidism |
1 |
3 |
a Fewer than 1% had parotid, pulmonary artery, breast, or lower genitourinary (urethra, cervix, vagina, testicular) involvement.
Skin lesions (46% of patients) appear as papules, vesicles, palpable purpura, ulcers, or subcutaneous nodules; biopsy reveals vasculitis, granuloma, or both. Cardiac involvement (8% of patients) manifests as pericarditis, coronary vasculitis, or, rarely, cardiomyopathy. Nervous system manifestations (23% of patients) include cranial neuritis, mononeuritis multiplex, or, rarely, cerebral vasculitis and/or granuloma.
Renal disease (77% of patients) generally dominates the clinical picture and, if left untreated, accounts directly or indirectly for most of the mortality in this disease. Although it may smolder in some cases as a mild glomerulitis with proteinuria, hematuria, and red blood cell casts, it is clear that once clinically detectable renal functional impairment occurs, rapidly progressive renal failure usually ensues unless appropriate treatment is instituted.
While the disease is active, most patients have nonspecific symptoms and signs such as malaise, weakness, arthralgias, anorexia, and weight loss. Fever may indicate activity of the underlying disease but more often reflects secondary infection, usually of the upper airway.
Characteristic laboratory findings include a markedly elevated erythrocyte sedimentation rate (ESR), mild anemia and leukocytosis, mild hypergammaglobulinemia (particularly of the IgA class), and mildly elevated rheumatoid factor. Thrombocytosis may be seen as an acute-phase reactant. Approximately 90% of patients with active Wegener’s granulomatosis have a positive antiproteinase-3 ANCA. However, in the absence of active disease, the sensitivity drops to ~60-70%. A small percentage of patients with Wegener’s granulomatosis may have antimyeloperoxidase rather than antiproteinase-3 antibodies.
Patients with Wegener’s granulomatosis have been found to have an increased incidence of venous thrombotic events. Although routine anticoagulation for all patients is not recommended, a heightened awareness for any clinical features suggestive of deep venous thrombosis or pulmonary emboli is warranted.
Diagnosis
The diagnosis of Wegener’s granulomatosis is made by the demonstration of necrotizing granulomatous vasculitis on tissue biopsy in a patient with compatible clinical features. Pulmonary tissue offers the highest diagnostic yield, almost invariably revealing granulomatous vasculitis. Biopsy of upper airway tissue usually reveals granulomatous inflammation with necrosis but may not show vasculitis. Renal biopsy can confirm the presence of pauci-immune glomerulonephritis.
The specificity of a positive antiproteinase-3 ANCA for Wegener’s granulomatosis is very high, especially if active glomerulonephritis is present. However, the presence of ANCA should be adjunctive and, with very rare exceptions, should not substitute for a tissue diagnosis. False-positive ANCA titers have been reported in certain infectious and neoplastic diseases.
In its typical presentation, the clinicopathologic complex of Wegener’s granulomatosis usually provides ready differentiation from other disorders. However, if all the typical features are not present at once, it needs to be differentiated from the other vasculitides, Goodpasture’s syndrome, relapsing polychondritis (Chap. 12), tumors of the upper airway or lung, and infectious diseases such as histoplasmosis, mucocutaneous leishmaniasis, and rhinoscle-roma as well as noninfectious granulomatous diseases.
Of particular note is the differentiation from midline granuloma and upper airway neoplasms, which are part of the spectrum of midline destructive diseases. These diseases lead to extreme tissue destruction and mutilation localized to the midline upper airway structures including the sinuses; erosion through the skin of the face commonly occurs, a feature that is extremely rare in Wegener’s granulomatosis. Although blood vessels may be involved in the intense inflammatory reaction and necrosis, primary vasculitis is seen rarely. When systemic involvement occurs, it usually declares itself as a neoplastic process. In this regard, it is likely that midline granuloma is part of the spectrum of angiocentric immunoproliferative lesions. The latter are considered to represent a spectrum of postthymic T cell proliferative lesions and should be treated as such. The term idiopathic has been applied to midline granuloma when extensive diagnostic workup including multiple biopsies has failed to reveal anything other than inflammation and necrosis. Under these circumstances, it is possible that the tumor cells were masked by the intensive inflammatory response. Such cases have responded to local irradiation with 50 Gy (5000 rad). Upper airway lesions should never be irradiated in Wegener’s granulomatosis. Cocaine-induced tissue injury can be another important mimic of Wegener’s granulomatosis in patients who present with isolated midline destructive disease. ANCA that target human neutrophil elastase can be found in patients with cocaine-induced midline destructive lesions and can confound the differentiation from Wegener’s granulomatosis.
Wegener’s granulomatosis must also be differentiated from lymphomatoid granulomatosis, which is an Epstein-Barr virus-positive B cell proliferation that is associated with an exuberant T cell reaction. Lymphomatoid granulomatosis is characterized by lung, skin, CNS, and kidney involvement in which atypical lymphocytoid and plas-macytoid cells infiltrate nonlymphoid tissue in an angioinvasive manner. In this regard, it clearly differs from Wegener’s granulomatosis in that it is not an inflammatory vasculitis in the classic sense but an infiltration of vessels with atypical mononuclear cells; granuloma may be present in involved tissues. Up to 50% of patients may develop a true malignant lymphoma.
Treatment:
Wegener’s granulomatosis
CYCLOPHOSPHAMIDE INDUCTION FOR SEVERE DISEASE Wegener’s granulomatosis was formerly universally fatal, usually within a few months after the onset of clinically apparent renal disease. Glucocorticoids alone led to some symptomatic improvement,with little effect on the ultimate course of the disease. It has been well established that the most effective therapy in this disease is cyclophosphamide given in doses of 2 mg/kg per day orally together with glucocorticoids. The leukocyte count should be monitored closely during therapy, and the dosage of cyclophosphamide should be adjusted in order to maintain the count above 3000/μΙ, which generally maintains the neutrophil count at ~1500/μΙ. With this approach,clinical remission can usually be induced and maintained without causing severe leukopenia with its associated risk of infection. As it was originally studied, cyclophosphamide was continued for 1 year following the induction of complete remission and gradually tapered and discontinued thereafter.
At the initiation of therapy,glucocorticoids should be administered together with cyclophosphamide.This can be given as prednisone, 1 mg/kg per day initially (for the first month of therapy) as a daily regimen, with gradual conversion to an alternate-day schedule followed by tapering and discontinuation after ~6 months.
Using the above regimen, the prognosis of this disease is excellent; marked improvement is seen in >90% of patients, and complete remissions are achieved in 75% of patients. A number of patients who developed irreversible renal failure but who achieved subsequent remission on appropriate therapy have undergone successful renal transplantation.
Despite the dramatic remissions induced by the therapeutic regimen described above, long-term follow-up of patients has revealed that ~50% of remissions are later associated with one or more relapses. Reinduction of remission is almost always achieved; however, a high percentage of patients ultimately have some degree of morbidity from irreversible features of their disease, such as varying degrees of renal insufficiency, hearing loss,tracheal stenosis,saddle nose deformity,and chronically impaired sinus function. The determination of relapse should be based on objective evidence of disease activity, taking care to rule out other features that may have a similar appearance such as infection, medication toxicity, or chronic disease sequelae. The ANCA titer can be misleading. Many patients who achieve remission continue to have elevated titers for years. In addition, one study found that >40% of patients who were in remission and had a fourfold increase in ANCA titer did not have a relapse in disease. Patients who relapse may not do so until many months or years after the rise in ANCA titer.Thus, a rise in ANCA titer by itself is not a harbinger of immediate disease relapse and should not lead to reinstitution or increase in immunosuppressive therapy. However, such a finding should prompt the clinician to examine the patient carefully for any objective evidence of active disease and to monitor that patient closely.
Certain types of morbidity are related to toxic side effects of treatment. Glucocorticoid-related side effects can include diabetes mellitus, cataracts, life-threatening infectious disease complications, serious osteoporosis, and severe cushingoid features.The risk of such toxicities can be reduced with the use of an alternate-day glucocorticoid regimen as outlined in the preceding regimen. Cyclophosphamide-related toxicities are more frequent and severe.Cystitis to varying degrees occurs in at least 30% of patients, bladder cancer in 6%, myelodysplasia in 2%, and there is a high risk of permanent infertility in both men and women.
Some reports have indicated therapeutic success with less frequent and severe toxic side effects using intermittent boluses of IV cyclophosphamide in place of daily administration. However, we and others have found an increased rate of relapse with bolus IV cyclophosphamide. We therefore recommend that the drug be given as daily oral therapy.
In patients with severe disease a regimen of daily cyclophosphamide and glucocorticoids is clearly the treatment of choice to induce remission. In the setting of rapidly progressive glomerulonephritis, the addition of plasmapheresis has been found to increase the rate of renal recovery. After patients have achieved remission, which typically occurs within 3-6 months, cyclophosphamide should be stopped and switched to methotrexate or azathioprine for remission mainte-nance.This approach is aimed at lessening the toxicity associated with chronic cyclophosphamide therapy. Methotrexate is administered orally or subcutaneously starting at a dosage of 0.3 mg/kg as a single weekly dose, not to exceed 15 mg/week. If the treatment is well tolerated after 1-2 weeks, the dosage should be increased by 2.5 mg weekly up to a dosage of 20-25 mg/week and maintained at that level.This regimen is given for 2 years past remission, after which time it is tapered by 2.5 mg each month until discontinuation.To lessen toxicity, methotrexate is often given together with folic acid, 1 mg daily,or folinic acid,5-10 mg once a week 24 h following methotrexate. Azathioprine, 2 mg/kg per d, has also proven effective in maintaining remission following induction with daily cyclophosphamide. In a randomized trial, methotrexate and azathioprine had comparable rate of toxicity and relapse. Given this, the choice of which agent to use is based on toxicity profile, as methotrexate cannot be given to patients with renal insufficiency or chronic liver disease, relapse history,and individual patient factors. In patients who are unable to receive methotrexate or azathioprine or who have relapsed through such treatment, mycophenolate mofetil, 1000 mg twice a day, may also sustain remission following cyclophosphamide induction in selected patients.
METHOTREXATE INDUCTION FOR NONSEVERE DISEASE For selected patients whose disease is not immediately life threatening or in those patients who have experienced significant cyclophosphamide toxicity, methotrexate together with glucocorticoids given at the dosages described above may be considered as an alternative for initial therapy.
BIOLOGIC THERAPIES Biologic therapies have increasingly been investigated in Wegener’s granulomatosis. Etanercept,a dimeric fusion protein containing the 75-kDa TNF receptor bound to human IgG1,was not found to sustain remission when used adjunctively to standard therapy and should not be used in the treatment of Wegener’s granulomatosis. Favorable preliminary results with the use of rituximab (anti-CD20) in
Wegener’s granulomatosis have been reported. However, pending further investigation of this agent through rigorous prospective trials, rituximab should not be used in place of standard treatments that are of proven efficacy.
TRIMETHOPRIM-SULFAMETHOXAZOLE Although certain reports have indicated that trimethoprim-sulfamethoxazole (TMP-SMX) may be of benefit in the treatment of Wegener’s granulomatosis, there are no firm data to substantiate this, particularly in patients with serious renal and pulmonary disease. In a study examining the effect of TMP-SMX on relapse,decreased relapses were shown only with regard to upper airway disease,and no differences in major organ relapses were observed.TMP-SMX alone should never be used to treat active Wegener’s granulomatosis outside of the upper airway.
ORGAN-SPECIFIC TREATMENT Not all manifestations ofWegener’s granulomatosis require or respond to cytotoxic therapy. In managing non-major organ disease, such as that isolated to the sinus,joints, or skin, the risks of treatment should be carefully weighed against the benefits. Given the potential toxicities of this agent, treatment with cyclophosphamide is rarely if ever justified for the treatment of isolated sinus disease in Wegener’s granulomatosis. Although patients with non-major organ disease may be effectively treated without cytotoxic therapy, these individuals must be monitored closely for the development of disease activity affecting the lungs, kidneys, or other major organs. Sub-glottic tracheal stenosis and endobronchial stenosis are examples of disease manifestations that do not typically respond to systemic immunosuppressive treatment.