Systemic Risk Factors
Age is the most potent risk factor for OA, with prevalence and incidence of disease rising dramatically with age. Radiographic evidence of OA is rare in individuals under age 40; however, in some joints, such as the hands, OA occurs in >50% of persons over age 70. Aging increases joint vulnerability through several mechanisms. Whereas dynamic loading of joints stimulates cartilage matrix by chondrocytes in young cartilage, aged cartilage is less responsive to these stimuli. Indeed, because of the poor responsiveness of older cartilage to such stimulation, cartilage transplant operations are far more challenging in older than in younger persons. Partly because of this failure to synthesize matrix with loading, cartilage thins with age, and thinner cartilage experiences higher shear stress at basal layers and is at greater risk of cartilage damage.Aging also increases the likelihood of failure of major joint protectors. Muscles that bridge the joint become weaker with age and also respond less quickly to oncoming impulses. Sensory nerve input slows with age, retarding the feedback loop of mechanoreceptors to muscles and tendons related to their tension and position. Ligaments stretch with age, making them less able to absorb impulses. A combination of all of these factors works in concert to increase the vulnerability of older joints to OA.
Older women are at high risk of OA in all joints, a risk that emerges as women reach their sixth decade. While hormone loss with menopause may contribute to this risk, there is little understanding of the vulnerability of older women’s joints to OA.
Heritability and Genetics
OA is a highly heritable disease, but its heritability varies by joint. Fifty percent of the hand and hip OA in the community is attributable to inheritance, i.e., to disease present in other members of the family. However, the heritable proportion of knee OA is at most 30%, with some studies suggesting no heritability at all. Whereas many people with OA have disease in multiple joints, this “generalized OA” phenotype is rarely inherited and is more often a consequence of aging.
Emerging evidence suggests that persons with genetic mutations in proteins that regulate the transcription of major cartilage molecules are at high risk of OA. One gene implicated is FRZB, in which a mutation may put a woman at high risk of hip OA. FRZB is a gene for a Frizzle protein that antagonizes an extracellular Wnt ligand, and the Wnt signaling pathway plays a critical role in matrix synthesis and joint development.
Global Considerations
Hip OA is rare in China and in immigrants from China to the United States. However, OA in the knees is at least as common, if not more so, in Chinese than in Caucasians from the United States, and knee OA represents a major cause of disability in China. Anatomic differences between Chinese and Caucasian hips may account for much of the difference in prevalence, with Caucasian hips having a higher prevalence of anatomic predispositions to the development of OA. Persons from Africa, but not African Americans, may also have a very low rate of hip OA.
Risk Factors in the Joint Environment
Some risk factors increase vulnerability of the joint through local effects on the joint environment. With changes in joint anatomy, for example, load across the joint is no longer distributed evenly across the joint surface, but rather shows an increase in focal stress. In the hip, three uncommon developmental abnormalities occurring in utero or childhood, congenital dysplasia, Legg-Perthes disease, and slipped femoral capital epiphysis, leave a child with distortions of hip joint anatomy that often lead to OA later in life. Girls are predominantly affected by acetabular dysplasia, a mild form of congenital dislocation, whereas the other abnormalities more often affect boys. Depending on the severity of the anatomic abnormalities, hip OA occurs either in young adulthood (severe abnormalities) or middle age (mild abnormalities).
Major injuries to a joint also can produce anatomic abnormalities that leave the joint susceptible to OA. For example, a fracture through the joint surface often causes OA in joints in which the disease is otherwise rare such as the ankle and the wrist. Avascular necrosis can lead to collapse of dead bone at the articular surface, producing anatomic irregularities and subsequent OA.
Tears of ligaments that protect the joints, such as the anterior cruciate ligament in the knee and the labrum in the hip, can increase joint susceptibility and lead to premature OA. While meniscal tears may increase the risk of OA, meniscectomy operations, including selective ones, increase the risk of later disease, perhaps independent of the tear that led to the operation. Even injuries that do not produce diagnosed joint injuries may increase the risk of OA, perhaps because the structural injury was not detected at the time. For example, in the Framingham study subjects, men with a history of major knee injury, but no surgery, had a 3.5-fold increased risk for subsequent knee OA.
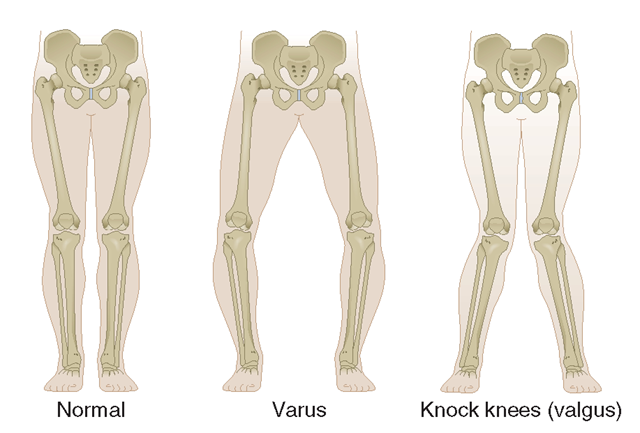
Another source of anatomic abnormality is malalignment across the joint (Fig. 18-5). This factor has been best studied in the knee, which is the fulcrum of the longest lever arm in the body.Varus (bowlegged) knees with OA are at exceedingly high risk of cartilage loss in the medial or inner compartment of the knee, whereas valgus (knock kneed) malalignment predisposes to rapid cartilage loss in the lateral compartment. Malalignment causes this effect by decreasing contact area during loading, increasing stress on a focal area or cartilage, which While it is likely that the weakness in muscles bridging a joint increases the risk of OA in that joint, there is no definitive evidence in this regard.
FIGURE 18-5
The two types of limb malalignment in the frontal plane: varus, in which the stress is placed across the medial compartment of the knee joint, and valgus, which places excess stress across the lateral compartment of the knee.
Patients with knee OA have impaired proprioception across their knees, and this may predispose them to further disease progression.The role of bone in serving as a shock absorber for impact load is not well understood, but persons with increased bone density are at high risk of OA, suggesting that the resistance of bone to impact during joint use may play a role in disease development.
Loading Factors
Obesity
Three to six times body weight is exerted across the knee during single leg stance. Any increase in weight may be multiplied by this factor to reveal the excess force across the knee in overweight persons during walking. Obesity is a well-recognized and potent risk factor for the development of knee OA and, less so, for hip OA. Obesity precedes the development of disease and is not just a consequence of the inactivity present in those with disease. Obesity is a stronger risk factor for disease in women than in men, and in women, the relationship of weight to the risk of disease is linear, so that with each increase in weight, there is a commensurate increase in risk. Weight loss in women lowers the risk of developing symptomatic disease. Not only is obesity a risk factor for OA in weight-bearing joints, but obese persons have more severe symptoms from the disease.
Obesity’s effect on the development and progression of disease is mediated mostly through the increased loading in weight-bearing joints that occurs in overweight persons. However, a modest association of obesity with an increased risk of hand OA suggests that there may be a systemic metabolic factor circulating in obese persons that affects disease risk also.
Repeated Use of Joint
There are two categories of repetitive joint use: occupational use and leisure time physical activities.Workers performing repetitive tasks as part of their occupations for many years are at high risk of developing OA in the joints they use repeatedly. For example, farmers are at high risk for hip OA, miners have high rates of OA in knees and spine, and shipyard and dockyard workers have a higher prevalence of disease in knees and fingers than do office workers. Even within a textile mill, women whose jobs required fine pincer grip [increasing the stress across the interphalangeal (IP) joints] had much more distal IP (DIP) joint OA than women of the same age whose jobs required repeated power grip, a motion that does not stress the DIP joints. Workers whose jobs require regular knee bending or lifting or carrying heavy loads have a high rate of knee OA. One reason why workers may get disease is that during long days at work, their muscles may gradually become exhausted, no longer serving as effective joint protectors.
While exercise is a major element of the treatment of OA, certain types of exercise may paradoxically increase the risk of disease. While recreational runners are not at increased risk of knee OA, studies suggest that they do have a modest increased risk of disease in the hip. However, persons who have already sustained major knee injuries are at increased risk of progressive knee OA as a consequence of running. Compared to nonrunners, elite runners (professional runners and those on Olympic teams) have high risks of both knee and hip OA. Given the widespread recommendation to adopt a healthier, more exercise-filled lifestyle; longitudinal epidemiologic studies of exercise contain cautionary notes. For example, women with increased levels of physical activity, either as teenagers or at age 50, had a higher risk of developing symptomatic hip disease later in life than women who were sedentary. Other athletic activities that pose high risks of joint injury, such as football, may thereby predispose to OA.
Pathology
The pathology of OA provides evidence of the panartic-ular involvement of disease. Cartilage initially shows surface fibrillation and irregularity. As disease progresses, focal erosions develop there, and these eventually extend down to the subjacent bone. With further progression, cartilage erosion down to bone expands to involve a larger proportion of the joint surface, even though OA remains a focal disease with nonuniform loss of cartilage (Fig. 18-6).
After an injury to cartilage, chondrocytes undergo mitosis and clustering. While the metabolic activity of these chondrocyte clusters is high, the net effect of this activity is to promote proteoglycan depletion in the matrix surrounding the chondrocytes. This is because the catabolic activity is greater than the synthetic. As disease develops, collagen matrix becomes damaged, the negative charges of proteoglycans get exposed, and cartilage swells from ionic attraction to water molecules. Because in damaged cartilage proteoglycans are no longer forced into close proximity, cartilage does not bounce back after loading as it did when healthy, and cartilage becomes vulnerable to further injury. Chondrocytes at the basal level of cartilage undergo apoptosis.
FIGURE 18-6
Pathologic changes of osteoarthritis in a toe joint. Note the nonuniform loss of cartilage (arrowhead vs solid arrow), the increased thickness of the subchondral bone envelope (solid arrow), and the osteophyte (open arrow).
With loss of cartilage come alterations in subchondral bone. Stimulated by growth factors and cytokines, osteoclasts and osteoblasts in the subchondral bony plate, just underneath cartilage, become activated. Bone formation produces a thickening and stiffness of the subchondral plate that occurs even before cartilage ulcerates. Trauma to bone during joint loading may be the primary factor driving this bone response, with healing from injury (including microcracks) producing stiffness. Small areas of osteonecrosis usually exist in joints with advanced disease. Bone death may also be caused by bone trauma with shearing of microvasculature, leading to a cutoff of vascular supply to some bone areas.
At the margin of the joint, near areas of cartilage loss, osteophytes form. These start as outgrowths of new cartilage and, with neurovascular invasion from the bone, this cartilage ossifies. Osteophytes are an important radiographic hallmark of OA. In malaligned joints, osteophytes grow larger on the side of the joint subject to the most loading stress (e.g., in varus knees, osteophytes grow larger on the medial side).
The synovium produces lubricating fluids that minimize shear stress during motion. In healthy joints, the synovium consists of a single discontinuous layer filled with fat and containing two types of cells—macrophages and fibroblasts—but, in OA, it can sometimes become edematous and inflamed. There is a migration of macrophages from the periphery into the tissue, and cells lining the synovium proliferate. Enzymes secreted by the synovium digest cartilage matrix that has been sheared from the surface of the cartilage.
Additional pathologic changes occur in the capsule, which stretches, becomes edematous, and can become fibrotic.
The pathology of OA is not identical across joints. In hand joints with severe OA, for example, there are often cartilage erosions in the center of the joint probably produced by bony pressure from the opposite side of the joint. Bone remodeling is a prominent feature of hand OA, in part because of the thin cartilage in each hand joint. In hand OA, pathology has also been noted in ligament site insertions, which may help propagate disease.
Basic calcium phosphate and calcium pyrophosphate dihydrate crystals are present microscopically in most joints with end-stage OA. Their role in osteoarthritic cartilage is unclear, but their release from cartilage into the joint space and joint fluid likely triggers synovial inflammation, which can, in turn, produce release of enzymes and trigger nociceptive stimulation.
Sources of Pain
Because cartilage is aneural, cartilage loss in a joint is not accompanied by pain. Thus, pain in OA likely arises from structures outside the cartilage. Innervated structures in the joint include the synovium, ligaments, joint capsule, muscles, and subchondral bone. Most of these are not visualized by x-ray, and the severity of x-ray changes in OA correlates poorly with pain severity.
Based on MRI studies in osteoarthritic knees comparing those with and without pain, and on studies mapping tenderness in unanesthetized joints, likely sources of pain include synovial inflammation, joint effusions, and bone marrow edema. Modest synovitis develops in many but not all osteoarthritic joints. Some diseased joints have no synovitis, whereas others have synovial inflammation that approaches the severity ofjoints with rheumatoid arthritis (Chap. 5).The presence of synovitis on MRI is correlated with the presence and severity of knee pain. Capsular stretching from fluid in the joint stimulates nociceptive fibers there, inducing pain. Increased focal loading as part of the disease not only damages cartilage but probably also injures the underlying bone. As a consequence, bone marrow edema appears on the MRI; histologically, this edema may signal the presence of microcracks and scar, which are the consequences of trauma. These lesions may stimulate bone nociceptive fibers. Also, hemostatic pressure within bone rises in OA, and the increased pres- ‘ sure itself may stimulate nociceptive fibers, causing pain. Lastly, osteophytes themselves may be a source of pain. When osteophytes grow, neurovascular innervation penetrates through the base of the bone into the cartilage and into the developing osteophyte.
Pain may arise from outside the joint also, including bursae near the joints. Common sources of pain near the knee are anserine bursitis and iliotibial band syndrome.
Clinical Features
Joint pain from OA is activity related. Pain comes on either during or just after joint use and then gradually resolves. Examples include knee or hip pain with going up or down stairs, pain in weight-bearing joints when walking, and, for hand OA, pain after cooking. Early in disease, pain is episodic, triggered often by a day or two of overactive use of a diseased joint, such as a person with knee OA taking a long run and noticing a few days of pain thereafter. As disease progresses, the pain becomes continuous and even begins to be bothersome at night. Stiffness of the affected joint may be prominent, but morning stiffness is usually brief (<30 min).
In knees, buckling may occur, in part due to weakness of muscles crossing the joint. Mechanical symptoms, such as buckling, catching, or locking, could also signify internal derangement, such as meniscal tears, and need to be evaluated. In the knee, pain with activities requiring knee flexion, such as stair climbing and arising from a chair, often emanates from the patellofemoral compartment of the knee, which does not actively articulate until the knee is bent ~35°.
OA is the most common cause of chronic knee pain in persons over age 45, but the differential diagnosis is long. Inflammatory arthritis is likely if there is prominent morning stiffness and many other joints are affected. Bursitis occurs commonly around knees and hips. A physical examination should focus on whether tenderness is over the joint line (at the junction of the two bones around which the joint is articulating) or is outside of it. Anserine bursitis, medial and distal to the knee, is an extremely common cause of chronic knee pain that may respond to a glucocorticoid injection. Prominent nocturnal pain in the absence of end-stage OA merits a distinct workup. For hip pain, OA can be detected by loss of internal rotation on passive movement, and pain isolated to an area lateral to the hip joint usually reflects the presence of trochanteric bursitis.
No blood tests are routinely indicated for workup of patients with OA unless symptoms and signs suggest inflammatory arthritis. Examination of the synovial fluid is often more helpful diagnostically than an x-ray. If the synovial fluid white count is >1000^L, inflammatory arthritis or gout or pseudogout are likely, the latter two being also identified by the presence of crystals.
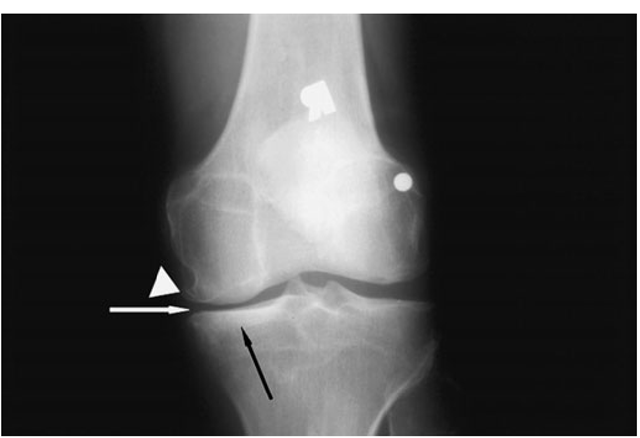
FIGURE 18-7
X-ray of knee with medial osteoarthritis. Note the narrowed joint space on medial side of the joint only (white arrow), the sclerosis of the bone in the medial compartment providing evidence of cortical thickening (black arrow), and the osteophytes in the medial femur (white wedge).
X-rays are indicated to evaluate chronic hand pain and hip pain thought to be due to OA, as the diagnosis is often unclear without confirming radiographs. For knee pain, x-rays should be obtained if symptoms or signs are not typical of OA or if knee pain persists after effective treatment. In OA, radiographic findings (Fig. 18-7) correlate poorly with the presence and severity of pain. Further, radiographs may be normal in early disease as they are insensitive to cartilage loss and other early findings.
While MRI may reveal the extent of pathology in an osteoarthritic joint, it is not indicated as part of the diagnostic workup. Findings such as meniscal tears in cartilage and bone lesions occur in most patients with OA in the knee, but almost never warrant a change in therapy.