Osteoarthritis (OA) is the most common type of arthritis. Its high prevalence, especially in the elderly, and the high rate of disability related to disease make it a leading cause of disability in the elderly. Because of the aging ofWestern populations and because obesity, a major risk factor, is increasing in prevalence, the occurrence of osteoarthritis is on the rise. In the United States, osteoarthritis prevalence will increase from 66-100% by the year 2020.
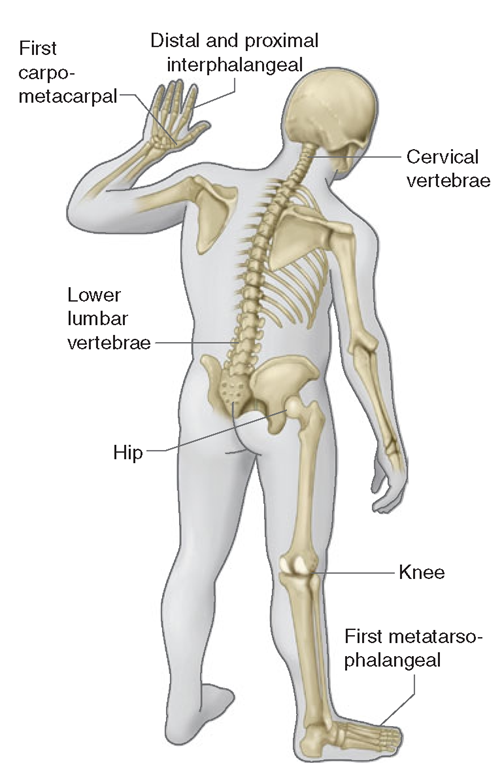
OA affects certain joints,yet spares others (Fig. 18-1). Commonly affected joints include the cervical and lumbosacral spine, hip, knee, and first metatarsal phalangeal joint (MTP). In the hands, the distal and proximal inter-phalangeal joints and the base of the thumb are often affected. Usually spared are the wrist, elbow, and ankle. Our joints were designed in an evolutionary sense, when humans were still brachiating apes. We thus develop OA in joints that were ill designed for these tasks, i.e.,joints involved in pincer grip in the hands and lower extremity weight-bearing joints. Some joints, like the ankles, may be spared because their articular cartilage may be uniquely resistant to loading stresses.
OA can be diagnosed based on structural abnormalities or on the symptoms these abnormalities evoke. Based on cadaveric studies, structural changes of OA are nearly universal by the elderly years. These include cartilage loss (seen as joint space loss on x-rays) and osteophytes. Many persons with x-ray evidence of OA have no joint symptoms and, while the prevalence of structural abnormalities is of interest in understanding disease pathogenesis, what matters more from a clinical and public health perspective is the prevalence of symptomatic OA.
FIGURE 18-1
Joints affected by osteoarthritis.
Symptoms, usually joint pain, determine disability, visits to clinicians, and disease costs.
FIGURE 18-2
Severe osteoarthritis of the hands affecting the distal inter-phalangeal joints (Heberden’s nodes) and the proximal inter-phalangeal joints (Bouchard’s nodes). There is no clear bony enlargement of the other common site in the hands, the thumb base.
Symptomatic OA of the knee (pain on most days of a recent month in a knee plus x-ray evidence of OA in that knee) occurs in ~12% of persons age ^60 in the United States and 6% of all adults age ^30. Symptomatic hip OA is roughly one-third as common as disease in the knee. While radiographically evident hand OA and the appearance of bony enlargement in affected hand joints (Fig. 18-2) are extremely common in older persons, most cases are often not symptomatic. Even so, recent studies suggest that symptomatic hand OA occurs in ~10% of elderly individuals and often produces measurable physical functional limitation.
The prevalence of OA correlates strikingly with age. Regardless of how it is defined, OA is uncommon in adults under age 40 and highly prevalent in those over age 60. It is also a disease that, at least in middle-aged and elderly persons, is much more common in women than in men, and sex differences in prevalence increase with age.
X-ray evidence of OA is common in the lower back and neck, but back pain and neck pain have not been tied to findings of OA on x-ray.
Definition
OA is joint failure, a disease in which all structures of the joint have undergone pathologic change, often in concert. The pathologic sine qua non of disease is hyaline articular cartilage loss, present in a focal and, initially, nonuniform manner. This is accompanied by increasing thickness and sclerosis of the subchondral bony plate, by outgrowth of osteophytes at the joint margin, by stretching of the articular capsule, by mild synovitis in many affected joints, and by weakness of muscles bridging the joint. In knees, meniscal degeneration is part of the disease. There are numerous pathways that lead to joint failure, but the initial step is often joint injury in the setting of a failure of protective mechanisms.
Joint Protective Mechanisms and Their Failure
Joint protectors include joint capsule and ligaments, muscle, sensory afferents, and underlying bone. Joint capsule and ligaments serve as joint protectors by providing a limit to excursion, thereby fixing the range of joint motion.
Synovial fluid reduces friction between articulating cartilage surfaces, thereby serving as a major protector against friction-induced cartilage wear. This lubrication function depends on the molecule lubricin, a mucinous glycoprotein secreted by synovial fibroblasts whose concentration diminishes after joint injury and in the face of synovial inflammation.
The ligaments, along with overlying skin and tendons, contain mechanoreceptor sensory afferent nerves. These mechanoreceptors fire at different frequencies throughout a joint’s range of motion, providing feedback by way of the spinal cord to muscles and tendons. As a consequence, these muscles and tendons can assume the right tension at appropriate points in joint excursion to act as optimal joint protectors, anticipating joint loading.
Muscles and tendons that bridge the joint are key joint protectors. Their co-contractions at the appropriate time in joint movement provide the appropriate power and acceleration for the limb to accomplish its tasks. Focal stress across the joint is minimized by muscle contraction that decelerates the joint before impact and ensures that when joint impact arrives, it is distributed broadly across the joint surface.
The bone underneath the cartilage may also provide a shock-absorbing function, as it may give way subtly to an oncoming impulse load.
Failure of these joint protectors increases the risk of joint injury and OA. For example, in animals, OA develops rapidly when a sensory nerve to the joint is sectioned and joint injury is induced. Similarly, in humans, Charcot arthropathy, which is a severe and rapidly progressive OA, develops when minor joint injury occurs in the presence of posterior column peripheral neuropathy. Another example of joint protector failure is rupture of ligaments, a well-known cause of the early development of OA.
Cartilage and Its Role in Joint Failure
In addition to being a primary target tissue for disease, cartilage also functions as a joint protector. A thin rim of tissue at the ends of two opposing bones, cartilage is lubricated by synovial fluid to provide an almost friction-less surface across which the two bones move. The compressible stiffness of cartilage compared to bone provides the joint with impact-absorbing capacity. Both the smooth frictionless surface and the compressive stiffness of cartilage serve as protective mechanisms preventing joint injury.
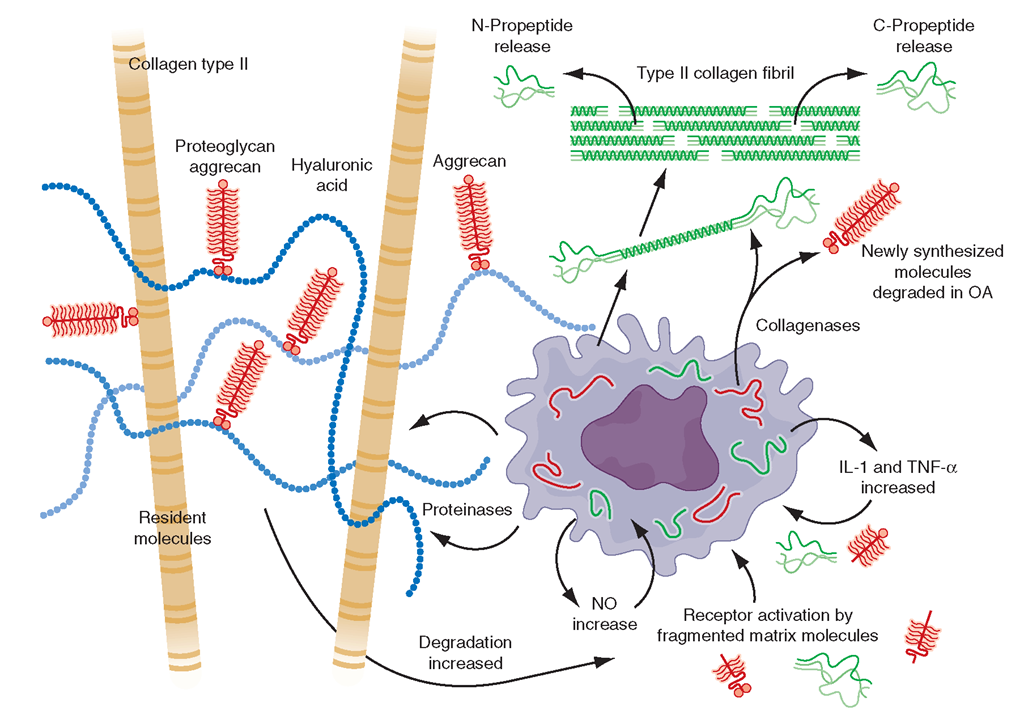
FIGURE 18-3
The chondrocyte and its products, type II collagen, aggre-can and enzymes which degrade these structures along with molecules stimulating chondrocytes.
Since the earliest changes of OA may occur in cartilage, and abnormalities there can accelerate disease development, understanding the structure and physiology of cartilage is critical to an appreciation of disease pathogenesis. The two major macromolecules in cartilage are type 2 collagen, which provides cartilage its tensile strength, and aggrecan, a proteoglycan macromolecule linked with hyaluronic acid, which consists of highly negatively charged glycosaminoglycans. In normal cartilage, type 2 collagen is woven tightly, constraining the aggrecan molecules in the interstices between collagen strands, forcing these highly negatively charged molecules into close proximity with one another. The aggrecan molecule, through electrostatic repulsion of its negative charges, gives cartilage its compressive stiffness. Chondrocytes, the cells within this avascular tissue, synthesize all elements of the matrix. In addition, they produce enzymes that break down the matrix and cytokines and growth factors, which in turn provide autocrine/paracrine feedback that modulates synthesis of matrix molecules (Fig. 18-3). Cartilage matrix synthesis and catabolism are in a dynamic equilibrium influenced by the cytokine and growth factor environment and by mechanical stress. While chondrocytes synthesize numerous enzymes, especially matrix metallo-proteinases (MMPs), there are only a few that are critical molecules stimulating chondrocytes. [From AR Poole et al: Ann Rheum Dis 61 (S): ii78, 2002.] in regulating cartilage breakdown. Type 2 cartilage is degraded primarily by MMP-13 (collagenase 3), with other collagenases playing a minor role. Aggrecan degradation is complex but appears to be a consequence, in part, of activation of aggrecanase 1 (ADAMTS-4) and perhaps of MMPs. Both collagenase and aggrecanase act primarily in the territorial matrix surrounding chondrocytes; however, as the osteoarthritic process develops, their activities and effects spread throughout the matrix, especially in the superficial layers of cartilage.
The synovium and chondrocytes synthesize numerous growth factors and cytokines. Chief among them is interleukin (IL) 1, which exerts transcriptional effects on chondrocytes, stimulating production of proteinases and suppressing cartilage matrix synthesis. In animal models of OA, IL-1 blockade prevents cartilage loss. Tumor necrosis factor (TNF) α may play a similar role to that of IL-1. These cytokines also induce chondrocytes to synthesize prostaglandin E2, nitric oxide, and bone mor-phogenic protein 2 (BMP-2), which together have complex effects on matrix synthesis and degradation. Nitric oxide inhibits aggrecan synthesis and enhances proteinase activity, whereas BMP-2 is a potent stimulator of anabolic activity. At early stages in the matrix response to injury and in the healthy response to loading, the net effect of cytokine stimulation may be matrix turnover but, ultimately, excess IL-1 triggers a process of matrix degradation. Enzymes in the matrix are held in check by activation inhibitors, including tissue inhibitor of metal-loproteinase (TIMP). Growth factors are also part of this complex network, with insulin-like growth factor type 1 and transforming growth factor β playing prominent roles in stimulating anabolism by chondrocytes.
While healthy cartilage is metabolically sluggish, with slow matrix turnover and a net balance of synthesis and degradation, cartilage in early OA or after an injury is highly metabolically active. In the latter situation, stimulated chondrocytes synthesize enzymes and new matrix molecules, with those enzymes becoming activated in the matrix, causing release of degraded aggrecan and type 2 collagen into cartilage and into the synovial fluid. OA cartilage is characterized by gradual depletion of aggrecan, an unfurling of the tightly woven collagen matrix, and loss of type 2 collagen. With these changes comes increasing vulnerability of cartilage, which no longer has compressive stiffness.
Risk Factors
Joint vulnerability and joint loading are the two major factors contributing to the development of OA. On the one hand, a vulnerable joint whose protectors are dysfunctional can develop OA with minimal levels of loading, perhaps even levels encountered during everyday activities. On the other hand, in a young joint with competent protectors, a major acute injury or long-term overloading is necessary to precipitate disease. Risk factors for OA can be understood in terms of their effect either on joint vulnerability or on loading (Fig. 18-4).
FIGURE 18-4
Risk factors for osteoarthritis either contribute to the susceptibility of the joint (systemic factors or factors in the local joint environment) or they increase risk by the load they put on the joint. Usually a combination of loading and susceptibility factors is required to cause disease or its progression.