Introduction
Although Staphylococcus aureus, Neisseria gonorrhoeae, and other bacteria are the most common causes of infectious arthritis, various mycobacteria, spirochetes, fungi, and viruses also infect joints (Table 20-1). Since acute bacterial infection can rapidly destroy articular cartilage, all inflamed joints must be evaluated without delay to exclude noninfectious processes and to determine appropriate antimicrobial therapy and drainage procedures. For more detailed information on infectious arthritis due to specific organisms, the reader is referred to the topics on those organisms.
Acute bacterial infection typically involves a single joint or a few joints. Subacute or chronic monarthritis or oligoarthritis suggests mycobacterial or fungal infection; episodic inflammation is seen in syphilis, Lyme disease, and the reactive arthritis that follows enteric infections and chlamydial urethritis. Acute polyarticular inflammation occurs as an immunologic reaction during the course of endocarditis, rheumatic fever, disseminated neisserial infection, and acute hepatitis B. Bacteria and viruses occasionally infect multiple joints, the former most commonly in persons with rheumatoid arthritis.
Approach to the Patient: Infectious Arthritis
Aspiration of synovial fluid—an essential element in the evaluation of potentially infected joints—can be performed without difficulty in most cases by the insertion of a large-bore needle into the site of maximal fluctuance or tenderness or by the route of easiest access. Ultrasonography or fluoroscopy may be used to guide aspiration of difficult-to-localize effusions of the hip and, occasionally, the shoulder and other joints. Normal synovial fluid contains <180 cells (predominantly mononuclear cells) per microliter. Synovial cell counts averaging 100,000^L (range, 25,000-250,000^L),with >90% neutrophils, are characteristic of acute bacterial infections. Crystal-induced, rheumatoid, and other nonin-fectious inflammatory arthritides are usually associated with <30,000-50,000 cells^L; cell counts of 10,000-30,000^L, with 50-70% neutrophils and the remainder lymphocytes, are common in mycobacterial and fungal infections. Definitive diagnosis of an infectious process relies on identification of the pathogen in stained smears of synovial fluid, isolation of the pathogen from cultures of synovial fluid and blood, or detection of microbial nucleic acids and proteins by polymerase chain reaction (PCR)-based assays and immunologic techniques.
Acute Bacterial Arthritis
Pathogenesis
Bacteria enter the joint from the bloodstream; from a contiguous site of infection in bone or soft tissue; or by direct inoculation during surgery, injection, animal or human bite, or trauma.
TABLE 20-1
|
DIFFERENTIAL DIAGNOSIS OF ARTHRITIS SYNDROMES |
|
|
|
ACUTE MONARTICULAR ARTHRITIS |
CHRONIC MONARTICULAR ARTHRITIS |
POLYARTICULAR ARTHRITIS |
|
Staphylococcus aureus |
Mycobacterium tuberculosis |
Neisseria meningitidis |
|
Streptococcus pneumoniae |
Nontuberculous mycobacteria |
N. gonorrhoeae |
|
β-Hemolytic streptococci |
Borrelia burgdorferi |
Nongonococcal bacterial arthritis |
|
Gram-negative bacilli |
Treponema pallidum |
Bacterial endocarditis |
|
Neisseria gonorrhoeae |
Candida species |
Candida species |
|
Candida species |
Sporothrix schenckii |
Poncet’s disease (tuberculous |
|
Crystal-induced arthritis |
Coccidioides immitis |
rheumatism) |
|
Fracture |
Blastomyces dermatitidis |
Hepatitis B virus |
|
Hemarthrosis |
Aspergillus species |
Parvovirus B19 |
|
Foreign body |
Cryptococcus neoformans |
HIV |
|
Osteoarthritis |
Nocardia species |
Human T-lymphotropic virus |
|
Ischemic necrosis |
Brucella species |
type I |
|
Monarticular rheumatoid arthritis |
Legg-Calvé-Perthes disease |
Rubella virus |
|
Osteoarthritis |
Arthropod-borne viruses Sickle cell disease flare Reactive arthritis Serum sickness Acute rheumatic fever Inflammatory bowel disease Systemic lupus erythematosus Rheumatoid arthritis/Still’s disease Other vasculitides Sarcoidosis |
|
In hematogenous infection, bacteria escape from synovial capillaries, which have no limiting basement membrane, and within hours provoke neutrophilic infiltration of the synovium. Neutrophils and bacteria enter the joint space; later, bacteria adhere to articular cartilage. Degradation of cartilage begins within 48 h as a result of increased intraarticular pressure, release of proteases and cytokines from chondrocytes and synovial macrophages, and invasion of the cartilage by bacteria and inflammatory cells. Histologic studies reveal bacteria lining the synovium and cartilage as well as abscesses extending into the synovium, cartilage, and—in severe cases—subchondral bone. Synovial proliferation results in the formation of a pannus over the cartilage, and thrombosis of inflamed synovial vessels develops. Bacterial factors that appear important in the pathogenesis of infective arthritis include various surface-associated adhesins in S. aureus that permit adherence to cartilage and endotoxins that promote chondrocyte-mediated breakdown of cartilage.
Microbiology
The hematogenous route of infection is the most common route in all age groups, and nearly every bacterial pathogen is capable of causing septic arthritis. In infants, group B streptococci, gram-negative enteric bacilli, and S. aureus are the usual pathogens. Since the advent of the Haemophilus influenzae vaccine, S. aureus, Streptococcus pyogenes (group A Streptococcus), and (in some centers) Kingella kingae have predominated among children <5 years of age. Among young adults and adolescents, N. gonorrhoeae is the most commonly implicated organism. S. aureus accounts for most nongonococcal isolates in adults of all ages; gram-negative bacilli, pneumococci, and β-hemolytic streptococci—particularly groups A and B, but also groups C, G, and F—are involved in up to one-third of cases in older adults, especially those with underlying comorbid illnesses.
Infections after surgical procedures or penetrating injuries are due most often to S. aureus and occasionally to other gram-positive bacteria or gram-negative bacilli. Infections with coagulase-negative staphylococci are unusual except after the implantation of prosthetic joints or arthroscopy. Anaerobic organisms, often in association with aerobic or facultative bacteria, are found after human bites and when decubitus ulcers or intraabdominal abscesses spread into adjacent joints. Polymicrobial infections complicate traumatic injuries with extensive contamination. Bites and scratches from cats and other animals may introduce Pasteurella multocida into joints, and bites from humans may introduce Eikenella corrodens or other components of the oral flora.
Nongonococcal Bacterial Arthritis
Epidemiology
Although hematogenous infections with virulent organisms such as S. aureus, H. influenzae, and pyogenic streptococci occur in healthy persons, there is an underlying host predisposition in many cases of septic arthritis. Patients with rheumatoid arthritis have the highest incidence of infective arthritis (most often secondary to S. aureus) because of chronically inflamed joints; glucocorticoid therapy; and frequent breakdown of rheumatoid nodules, vasculitic ulcers, and skin overlying deformed joints. Diabetes mellitus, glucocorticoid therapy, hemodialysis, and malignancy all carry an increased risk of infection with S. aureus and gram-negative bacilli. Tumor necrosis factor (TNF) inhibitors (etanercept and infliximab), increasingly used for the treatment of rheumatoid arthritis, predispose to mycobacterial infections and possibly to other pyogenic bacterial infections and could be associated with septic arthritis in this population. Pneumococcal infections complicate alcoholism, deficiencies ofhumoral immunity, and hemoglobinopathies. Pneumococci, Salmonella, and H. influenzae cause septic arthritis in persons infected with HIV Persons with primary immunoglobulin deficiency are at risk for mycoplasmal arthritis, which results in permanent joint damage if treatment with tetracycline and IV immunoglobulin (IVIg) replacement is not administered promptly. IV drug users acquire staphylococcal and streptococcal infections from their own flora and acquire pseudomonal and other gram-negative infections from drugs and injection paraphernalia.
Clinical Manifestations
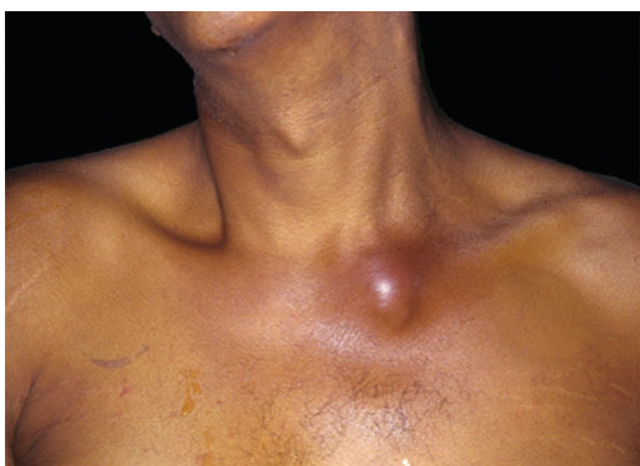
Some 90% of patients present with involvement of a single joint—most commonly the knee; less frequently the hip; and still less often the shoulder, wrist, or elbow. Small joints of the hands and feet are more likely to be affected after direct inoculation or a bite. Among IV drug users, infections of the spine, sacroiliac joints, or sternoclavicular joints (Fig. 20-1) are more common than infections of the appendicular skeleton. Polyarticular infection is most common among patients with rheumatoid arthritis and may resemble a flare of the underlying disease.
The usual presentation consists of moderate to severe pain that is uniform around the joint, effusion, muscle spasm, and decreased range of motion. Fever in the range of 38.3°-38.9°C (101°-102°F) and sometimes higher is common but may be lacking, especially in persons with rheumatoid arthritis, renal or hepatic insufficiency, or conditions requiring immunosuppressive therapy. The inflamed, swollen joint is usually evident on examination except in the case of a deeply situated joint, such as the hip, shoulder, or sacroiliac joint. Cellulitis, bursitis, and acute osteomyelitis, which may produce a similar clinical picture, should be distinguished from septic arthritis by their greater range of motion and less-than-circumferential swelling. A focus of extraarticular infection, such as a boil or pneumonia, should be sought. Peripheral-blood leukocytosis with a left shift and elevation of the erythrocyte sedimentation rate (ESR) or C-reactive protein level are common.
FIGURE 20-1
Acute septic arthritis of the sternoclavicular joint. A man in his 40s with a history of cirrhosis presented with a new onset of fever and lower neck pain. He had no history of IV drug use or previous catheter placement. Jaundice and a painful swollen area over his left sternoclavicular joint were evident on physical exam. Cultures of blood drawn at admission grew group B Streptococcus. The patient recovered after treatment with IV penicillin.
Plain radiographs show evidence of soft-tissue swelling, joint-space widening, and displacement of tissue planes by the distended capsule. Narrowing of the joint space and bony erosions indicate advanced infection and a poor prognosis. Ultrasound is useful for detecting effusions in the hip, and CT or MRI can demonstrate infections of the sacroiliac joint, the sternoclavicular joint, and the spine very well.
Laboratory Findings
Specimens of peripheral blood and synovial fluid should be obtained before antibiotics are administered. Blood cultures are positive in up to 50-70% of S. aureus infections but are less frequently positive in infections due to other organisms. The synovial fluid is turbid, serosan-guineous, or frankly purulent. Gram-stained smears confirm the presence of large numbers of neutrophils. Levels of total protein and lactate dehydrogenase in synovial fluid are elevated, and the glucose level is depressed; however, these findings are not specific for infection, and measurement of these levels is not necessary to make the diagnosis. The synovial fluid should be examined for crystals, because gout and pseudogout can resemble septic arthritis clinically, and infection and crystal-induced disease occasionally occur together. Organisms are seen on synovial fluid smears in nearly three-quarters of infections with S. aureus and streptococci and in 30-50% of infections due to gram-negative and other bacteria. Cultures of synovial fluid are positive in >90% of cases. Inoculation of synovial fluid into bottles containing liquid media for blood cultures increases the yield of culture, especially if the pathogen is a fastidious organism or the patient is taking an antibiotic. Although not yet widely available, PCR-based assays for bacterial DNA will also be useful for the diagnosis of partially treated or culture-negative bacterial arthritis.
Treatment:
Nongonococcal Bacterial Arthritis
Prompt administration of systemic antibiotics and drainage of the involved joint can prevent destruction of cartilage, postinfectious degenerative arthritis, joint instability, or deformity. Once samples of blood and synovial fluid have been obtained for culture, empirical antibiotics should be given that are directed against bacteria visualized on smears or against the pathogens that are likely, given the patient’s age and risk factors. Initial therapy should consist of the IV administration of bactericidal agents; direct instillation of antibiotics into the joint is not necessary to achieve adequate levels in synovial fluid and tissue. An IV third-generation cephalosporin such as cefotaxime (1 g every 8 h) or ceftriaxone (1-2 g every 24 h) provides adequate empirical coverage for most community-acquired infections in adults when smears show no organisms. Either oxacillin or nafcillin (2 g every 4 h) is used if there are gram-positive cocci on the smear. If methicillin-resistant S. aureus is a possible pathogen (e.g., when it is widespread in the community or in hospitalized patients), IV vancomycin (1 g every 12 h) should be given. In addition, an aminoglycoside or third-generation cephalosporin should be given to IV drug users or other patients in whom Pseudomonas aeruginosa may be the responsible agent.
Definitive therapy is based on the identity and antibiotic susceptibility of the bacteria isolated in culture. Infections due to staphylococci are treated with oxacillin, nafcillin, or vancomycin for 4 weeks. Pneumococcal and streptococcal infections due to penicillin-susceptible organisms respond to 2 weeks of therapy with penicillin G (2 million units IV every 4 h); infections caused by H. influenzae and by strains of Streptococcus pneumoniae that are resistant to penicillin are treated with cefotaxime or ceftriaxone for 2 weeks. Most enteric gramnegative infections can be cured in 3-4 weeks by a second- or third-generation cephalosporin given IV or by a fluoroquinolone,such as levofloxacin (500 mg IV or PO every 24 h). P.aeruginosa infection should be treated for at least 2 weeks with a combination regimen of an aminoglycoside plus either an extended-spectrum penicillin, such as mezlocillin (3 g IV every 4 h), or an antipseudomonal cephalosporin, such as ceftazidime (1 g IV every 8 h). If tolerated, this regimen is continued for an additional 2 weeks;alternatively,a fluoroquinolone, such as ciprofloxacin (750 mg PO twice daily), is given by itself or with the penicillin or cephalosporin in place of the aminoglycoside.
Timely drainage of pus and necrotic debris from the infected joint is required for a favorable outcome. Needle aspiration of readily accessible joints such as the knee may be adequate if loculations or particulate matter in the joint does not prevent its thorough decompression. Arthroscopic drainage and lavage may be employed initially or within several days if repeated needle aspiration fails to relieve symptoms, decrease the volume of the effusion and the synovial white cell count,and clear bacteria from smears and cultures. In some cases,arthrotomy is necessary to remove loculations and débride infected synovium, cartilage, or bone. Septic arthritis of the hip is best managed with arthrotomy, particularly in young children, in whom infection threatens the viability of the femoral head.Septic joints do not require immobilization except for pain control before symptoms are alleviated by treatment. Weight bearing should be avoided until signs of inflammation have subsided, but frequent passive motion of the joint is indicated to maintain full mobility. While addition of glucocorticoids to antibiotic treatment improves the outcome of S. aureus arthritis in experimental animals, no clinical trials have yet evaluated this approach in humans.
Gonococcal Arthritis
Epidemiology
Although its incidence has declined in recent years, gonococcal arthritis has accounted for up to 70% of episodes of infectious arthritis in persons <40 years of age in the United States. Arthritis due to N. gonorrhoeae is a consequence of bacteremia arising from gonococcal infection or, more frequently, from asymptomatic gonococcal mucosal colonization of the urethra, cervix, or pharynx. Women are at greatest risk during menses and during pregnancy and overall are two to three times more likely than men to develop disseminated gonococcal infection (DGI) and arthritis. Persons with complement deficiencies, especially of the terminal components, are prone to recurrent episodes of gonococcemia. Strains of gonococci that are most likely to cause DGI include those that produce transparent colonies in culture, have the type IA outer-membrane protein, or are of the AUH-auxotroph type.
Clinical Manifestations and Laboratory Findings
The most common manifestation of DGI is a syndrome of fever, chills, rash, and articular symptoms. Small numbers of papules that progress to hemorrhagic pustules develop on the trunk and the extensor surfaces of the distal extremities. Migratory arthritis and tenosynovitis of the knees, hands, wrists, feet, and ankles are prominent. The cutaneous lesions and articular findings are believed to be the consequence of an immune reaction to circulating gonococci and immune-complex deposition in tissues. Thus, cultures of synovial fluid are consistently negative, and blood cultures are positive in <45% of patients. Synovial fluid may be difficult to obtain from inflamed joints and usually contains only 10,00020,000 leukocytes^L.
True gonococcal septic arthritis is less common than the DGI syndrome and always follows DGI, which is unrecognized in one-third of patients. A single joint, such as the hip, knee, ankle, or wrist, is usually involved. Synovial fluid, which contains >50,000 leukocytes^L, can be obtained with ease; the gonococcus is only occasionally evident in gram-stained smears, and cultures of synovial fluid are positive in <40% of cases. Blood cultures are almost always negative.
Because it is difficult to isolate gonococci from synovial fluid and blood, specimens for culture should be obtained from potentially infected mucosal sites. Cultures and gram-stained smears of skin lesions are occasionally positive. All specimens for culture should be plated onto Thayer-Martin agar directly or in special transport media at the bedside and transferred promptly to the microbiology laboratory in an atmosphere of 5% CO2, as generated in a candle jar. PCR-based assays are extremely sensitive in detecting gonococcal DNA in synovial fluid. A dramatic alleviation of symptoms within 12-24 h after the initiation of appropriate antibiotic therapy supports a clinical diagnosis of the DGI syndrome if cultures are negative.