2. Molecular Tools for the Study of Early Xenopus Development
Figure 3. Neurula fate map and molecular anatomy. The fate map shown in the left panel defines four domains along the axis of the neural plate: forebrain, midbrain, hindbrain, and spinal chord (adapted from Eagelson and Harris (12)). The g right shows the expression of region-specific molecular markers within the neural plate. From left to right: the homeobo; marker of presumptive forebrain; Engrailed-2 (En2) is a homeobox gene that demarcates the midbrain-hindbrain junctioi factor Krox20 is a marker of rhombomers 3 and 5 of the hindbrain; the homeobox HoxB9 demarcates the presumptive sp.
The combination of experimental embryological approaches and molecular techniques has armed modern embryologists with powerful tools to dissect the molecular basis of early vertebrate development. Traditional lambda phage-based gene libraries are available from almost every stage of embryonic development, as well as from different regions of the embryo at a given stage. More recently, stage- and region-specific expression libraries have been developed that introduce a very powerful means to clone the factors responsible for a given activity. Expression cloning is discussed below (see section on Xenopus embryos for molecular studies). As with other animal model systems, screens for gain of function, loss of function, and modifier using expression libraries are available in Xenopus. For these approaches, nucleic acids or proteins (wild-type or mutated forms) are microinjected, either alone or simultaneously with pools of clones from cDNA libraries, into the oocytes, eggs, or blastulae (17). Injected oocytes and embryos are allowed to develop to the desired stage, after which they are submitted to phenotypic, histological, and molecular analysis. While gain-of-function experiments use ectopic expression of wild-type or constitutively active forms of the proteins encoded by the injected DNA or RNA, loss-of-function experiments utilize dominant-negative proteins or injections of antisense RNA, antisense DNA oligonucleotides, ribozymes or neutralizing antibodies. An in-depth review of the use of dominant-negative mutant approaches in embryology has been presented elsewhere (18). It is important to remember that the specificity of effects derived from antisense RNA, ribozymes, or antibody injections need to be interpreted with extreme caution. It is also important to control for the in vivo stability of micro-injected reagents.
2.1. Microinjection of DNA
Up to 100 pg of supercoiled, nicked, or linear DNA can be microinjected into oocytes or early embryos (17). The DNA topology has no effect on expression. In oocytes, the size of the nucleus (germinal vesicle) allows direct injection of the DNA into the nucleus. This type of microinjection has been used for transcriptional studies, as well as examination of RNA splicing and DNA recombination. In embryos, transcription begins when the blastula reaches about 4000 cells (see text above); consequently, injected DNA is not transcribed for the first few hours of development. Because of this property, injection of DNA, rather than RNA, is sometimes used to study later developmental events. The injected DNA is stable until gastrula-early neurula stages, after which it gradually decays. For reasons that are not well understood, only a fraction of the embryonic blastomeres that inherit the DNA will ultimately express it; thus, expression driven from injected DNA is always mosaic. As is the case with genetic studies, mosaic expression has its own advantages when contrasted with uniform expression.
The requirement for promoters is not very stringent in Xenopus laevis: most vertebrate and virus promoters work well in oocytes or embryos. However, with a few exceptions, tissue-specific expression cannot be achieved in this type of transient expression experiment. Another type of DNA injection involves the injection of single-stranded antisense oligonucleotides. In these experiments, regular or chemically modified antisense oligonucleotides are microinjected in the oocyte or embryos with the aim of binding to and eliminating the function of endogenous, sense mRNA.
2.2. Microinjection of RNA
The microinjected RNA can be of a biological origin, for example, poly bAb+ RNA isolated from different tissue samples. More frequently, however, single RNA or a population of RNAs are generated synthetically by in vitro transcription. In this case, specific vectors that contain prokaryotic transcriptional promoters, such as those of SP6, T3, or T7 are used. In addition, these vectors usually contain short stabilizing 5 and 3 untranslated regions flanking the cloning sites. Some vectors also add a polyA-polyC tail to the 3′ end of transcripts to increase their stability (17). Synthetic RNAs obtained by in vitro transcription of a single clone or pools of clones from expression libraries can be injected into oocytes and embryos. In oocytes, up to 50 ng of RNA can be injected, while embryos can tolerate up to 5 ng of RNA. Injected RNA or DNA may encode either wild-type or mutant versions of proteins. These mutant proteins can include constitutively active forms of known proteins, for example, constitutively active receptors, signal transducers, or transcription factors, which will activate signaling pathways or target genes in a ligand-independent fashion. Alternatively, they can encode dominant-negative versions of embryologically active proteins to generate loss of function phenotypes.
2.3. Microinjection of Proteins
Finally, injection of proteins, such as immunoglobulins, have also been reported in Xenopus embryos (19, 20). In these experiments, neutralizing antibodies against specific proteins are microinjected with the aim of eliminating the function of the endogenous protein. Again, as in the case with antisense techniques, interpretation of phenotypes should done with great care. Rescue experiments, in which the antigen and antibody are coinjected, provide the most stringent control for specificity.
3. The Xenopus Oocyte
Because of its size, the ease with which it can be obtained, and the relative ease with which it can be cultured, the Xenopus oocyte has become popular with researchers with a wide variety of interests. The fully mature Xenopus laevis oocyte is a large cell of about 1 mm in diameter. The oocyte is surrounded by a vitelline membrane and several layers of follicular cells. For most experimental conditions, the latter are removed, either mechanically or by enzymatic treatment, for example with collagenase. These defolliculated oocytes still have the vitelline membrane attached, which provides mechanical stability.
Oocytes can be used for a variety of functional studies, a few of which are described below. Up to 50 ng in a total of 50 nL of RNA can be microinjected into the cytoplasm of the oocyte. Unlike early embryos, the Xenopus oocyte is transcriptionally active. For DNA injections, most, if not all, mammalian promoters work well for expression in the context of the oocyte. Similarly, the polyadenylation signals from SV40 virus or mammals function well in the frog. Up to 10 ng of DNA can be injected directly into the germinal vesicle (nucleus) of the oocyte.
The Xenopus oocyte expression system has been used successfully for expression cloning, the study of individual proteins, and large-scale production of secreted proteins. The success of expression cloning depends on both the quality of the library and the specificity of the assay. For example, membrane proteins such as ion channels have been cloned by injecting oocytes with populations of RNA or DNA, followed by electrophysiological analysis to determine whether the desired channel is present in the pool. Further sib-selection of active fractions leads to the isolation of the clone encoding the channel. For isolation of receptors for hormones or growth factors, similar approaches can be used: binding of a labeled ligand is used to identify positive fractions.
Alternatively, individual cell-surface proteins can also be studied by injection of single RNA or cDNA. This has traditionally been used to study mutant forms of channels and receptors. Finally, although most oocyte experiments involve the analysis of membrane proteins, the Xenopus oocyte has been used more recently for the production of large amounts of secreted factors. In fact, one of the most efficient ways to obtain large quantities of highly-purified secreted growth factors, from any source, is to harvest the conditioned medium of oocytes injected with synthetic RNA encoding these factors.
Because of its size, the oocyte is also one of the favorite systems used by cell biologists interested in subcellular trafficking. Thus, trafficking between the endoplasmic reticulum and the Golgi apparatus in the secretory pathway, nuclear translocation, and subcellular localization of RNA have all been explored using Xenopus oocytes (for a detailed review, see Methods in Cell Biology,1992).
4. Use of Xenopus embryos and Embryonic Explants in Molecular Biology
Examples of a few assays that are commonly used in Xenopus to identify molecular players involved in embryological functions are presented here. It is important to emphasize that, while these techniques are widely used, new approaches emerge continuously that are custom fitted to address specific question.
4.1. Embryonic Explants
Specification studies combined with the analysis of cell-type-specific markers have provided a simple but powerful means of identifying factors involved in embryonic development. Explants can be cultured alone or in the presence of factors; alternatively, explants from different regions of the embryo of the same stage or different stages can be recombined to assess their influence on each other, as illustrated by the following examples for each case.
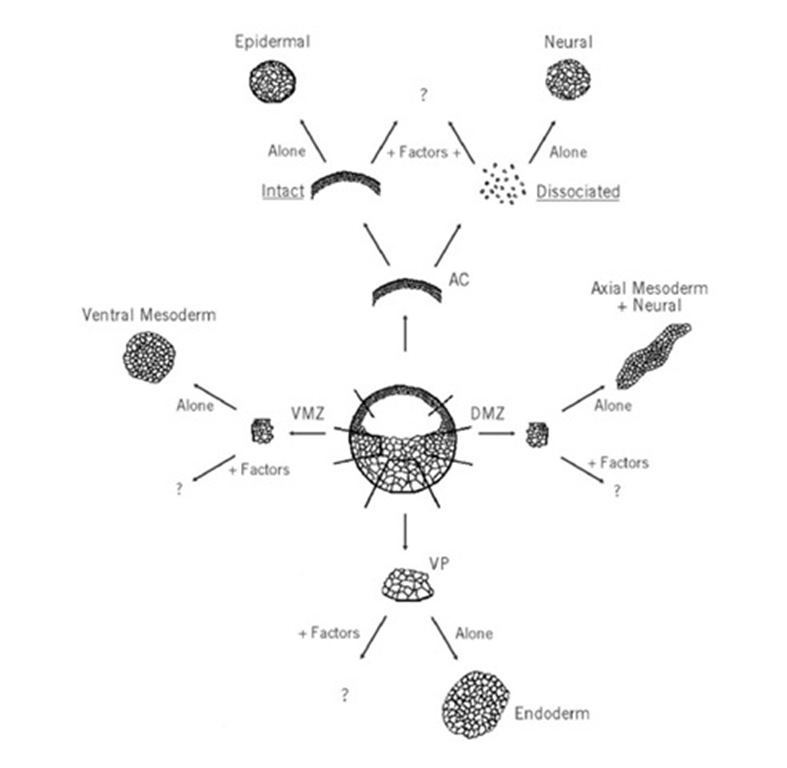
Although many type of explants can be derived from different regions of the amphibian embryo at various times of development, the most frequently used explants are derived from the early blastula or gastrula (Fig. 4). There are three types of explants traditionally used: from the ectoderm (or animal caps), mesoderm (ventral marginal zone, lateral marginal zone, and dorsal marginal zone), and endoderm (or vegetal poles).
Figure 4. Embryonic explants. A schematic cross section of a late blastula stage embryo (~4000 cells) is shown in the center. When the animal cap (AC) is removed and kept intact, it will differentiate as epidermis; if dissociated, it will give rise to neural tissue. The explanted dorsal marginal zone (DMZ) cultured in isolation will form axial mesoderm, such as notochord and somites, as well as neural tissue. The vegetal pole (VP) differentiates as endoderm (gut) in culture. The ventral marginal zone (VMZ) will form ventral mesoderm derivatives, such as blood cells, in culture. The behavior of these explants can be challenged in culture by the addition of molecular factors. These factors can be added to the explant culture after its isolation, or they can be presented as injected RNA or DNA at early stages of development by microinjection in the appropriate region of the embryo.
The most popular explant assay is the animal cap assay. When explanted at blastula or early gastrula stages and cultured alone, animal cap ectoderm differentiates as epidermis (Fig. 4). However, these early animal cap cells are pluripotent and are able to change their fate in response to exogenous factors. These factors can be provided as soluble protein directly incubated with the explants (21) or as synthetic RNA injected in the animal hemisphere during the first three embryonic cleavages (22).
The injected RNA remains confined to the site of injection, so one can target the RNA to different regions of the embryo. It was the use of Xenopus laevis animal caps that first allowed the characterization of molecular candidates involved in embryonic induction. A variant of the same assay involves the dissociation of the animal cap explants in Ca – and Mg -free medium. When the cells of the animal cap are dissociated and maintained for several hours in culture, they all adopt a neural fate (Fig. 4 (23). The type of neural tissue obtained by dissociation of animal cap explants is always anterior in character, including the cement gland (24). Animal caps assays have been used to answer a variety of questions concerning the study of inducers, morphogens (which are substances that elicit different fates at different concentration), and modifiers (factors that cannot induce specific cell fates on their own but have the ability to modify the character of cell types induced by other factors) (25). For example, when varying concentrations of the growth factor activin (which belongs to the transforming growth factor (TGF) b ligand superfamily) are applied to dissociated cells, it can induce mesoderm and endoderm in intact animal caps and can display morphogen-like properties. At low concentrations, activin will induce ventral mesoderm; at higher thresholds, axial mesoderm and ultimately endoderm. Growth factors belonging to the Wnt superfamily act instead as modifiers; they cannot induce mesodermal cell fates on their own, but they can change the type of mesoderm induced by activin. Inherent to an understanding of inducers and modifiers is the concept of competence, which relates to the window of time during which a given population of cells can respond to an inductive signal. The animal cap has also provided a means to address competence. Even though our knowledge of competence at the molecular level remains sparse, factors such as fibroblast growth factor (FGF) have been suggested to regulate the capacity of embryonic cells to respond to mesoderm inducers such as activin (25). It is important to note that animal cap explants have dorsal-ventral polarity (imposed upon them during the first cell cycle by cortical rotation), which may influence the response of these cells to inducers or modifiers. This polarity can be eliminated by using animal caps derived from hyperventralized or hyperdorsalized embryos.
Intact ventral marginal zone explants develop as ventral mesodermal cell types, including those of blood; once dissected, these explants adopt a rough spherical shape in culture. Dorsal marginal zone explants, however, elongate in culture and produce axial mesoderm, such as notochord and somites, as well as neural tissue. Ventral and dorsal marginal zone explants are useful for the assessment of the modifier activity of molecular vis-a-vis mesodermal patterning. Substances that can change the fate of these explants are good candidate modifiers.
Finally, vegetal pole explants of blastula or early gastrula embryo give rise exclusively to endoderm. These explants have been used to assess induction, patterning, and competence in the context of endoderm formation and have been gaining in popularity of late.
4.2. Recombinant Explants
Explants can also be recombined in different ways to evaluate a wide variety of embryological activities, such as induction, patterning, and cell autonomy. One of the most informative recombinant explants was originally performed by Nieuwkoop in 1969 (26). It was known that animal cap explants cultured alone give rise to only ectoderm (epidermis) and that vegetal pole explants give rise solely to endoderm (gut). Nieuwkoop showed that these explants cultured together also form mesoderm. This was the first demonstration of mesoderm induction in the vertebrate. Later experiments using lineage tracers (see text below) established that the vegetal pole was the source of the mesoderm-inducing signal; animal pole cells respond to these signals by changing their fate from ectoderm to mesoderm. Once it was established that the animal pole cells could respond to mesoderm-inducing signals, simple modifications of this assay allowed the molecular characterization of the molecular factors involved. The vegetal pole was replaced by conditioned medium from different cell lines or purified factors; the animal pole cells incubated in the presence of these factors were assayed for the formation of mesoderm.
In general, recombinant explants that generate more tissue types when recombined together than when cultured independently provide the basis for bioassays aimed at isolating the molecules involved. Similar approaches have also been used in the nervous system to recombine anterior and posterior neural plate tissue. Anterior neural plate explants, cultured alone, develop as forebrain, while posterior explants develop as spinal chord; when recombined, intermediate neural tissue also develops, including midbrain and hindbrain (27). Another famous explant system was introduced by Keller and co-workers. Known as "Keller" explants, they include both dorsal mesoderm and ectoderm, contacting each other only at a narrow boundary (28). These explants have been used to study the way planar inductive and patterning signals operate to generate the nervous system (29). It is also possible to recombine tissues of different age. For example, notochord from a neurula or tailbud can be sandwiched together with the animal cap of a blastula stage embryo (like a hotdog, where the bread is the animal cap and the meat is the notochord) to study vertical signals from the axial mesoderm that influence neural patterning in the ectoderm (30).
Heterospecific recombinant explants between Xenopus and chick and between Xenopus and mouse embryonic tissues have also been described (31, 32). In these cases, particular care should be taken to accommodate the physical requirements for the proper survival of the recombinants, such as temperature of incubation (different between the cold-blooded amphibian and the warm-blooded amniotes) and the ionic strength of the solution (50 mM salt for the amphibian tissue versus 150 mM for amniotes). The use of these heterospecies explants has led to the suggestion, for example, that the molecular natures of neural-inducing signals are conserved from amphibians to birds. Theoretically, therefore, an enormous variety of recombinant explants can be generated from amphibian tissue. The rationale behind their design depends solely on the questions asked and the creativity of the investigator.
4.3. Xenopus Embryos for Molecular Studies
In addition to the use of embryonic explants to isolate and characterize embryonic factors, expression cloning strategies have been successfully used in intact Xenopus embryos. In these approaches, fractionated cDNA libraries are subjected to in vitro transcription to generate synthetic RNA that is microinjected in different regions of either wild-type or perturbed embryos (see text below). Active fractions that generate a specific phenotype can be fractionated further by sib-selection until a single clone is obtained. These approaches have been extremely successful in isolating neural and mesodermal inducers and modifiers (33, 34). Injection in the animal pole, marginal zone, or vegetal pole of a one- or two-cell stage embryo will affect the differentiation of ectoderm, mesoderm and endoderm, respectively.
Expression libraries can also be used to rescue experimentally perturbed Xenopus embryos. Three types of perturbed Xenopus embryos are traditionally used: ventralized embryos, dorsalized embryos, and exogastrulae. In Xenopus, there is a link between the dorsal axis and anterior structures, as well as a link between the ventral and posterior structures (35). Ventralized embryos are obtained by UV irradiation of the vegetal pole during the first cell cycle (Fig. 5 ). This treatment blocks cortical rotation; thus, the dorsal side is never specified, and the embryo develops without a dorsal axis or head. Screening expression libraries based on their ability to rescue the ventralized phenotype of UV embryos has allowed the characterization of a large number of genes involved in the differentiation of dorsal structures. At the other end of the spectrum, incubation of the early blastula embryo (8- to 16-cell stage) in 300 mM LiCl will hyperdorsalize the embryo so that all ventral and posterior structures are eliminated (Fig. 5). In extreme cases, the embryo develops as a radially symmetrical head with retinal pigmented cells all around the circumference. Rescue of these embryos has been reported by injection of a single RNA, which suggests that these embryos could also be used for expression cloning strategies (33). Finally, exogastrulae represent another form of perturbed embryo in which, instead of invaginating, the cells of the gastrulae move out of the embryo (16, 36). Although these embryos have been used to study ectodermal and mesodermal interactions, they could also theoretically be used in expression cloning experiments, especially for screening genes involved in morphogenetic movements.
Figure 5. Experimentally perturbed Xenopus embryos. Varying amounts of exposure to UV irradiation during the first c< generates embryos that are gradually ventroposteriorized (left panels), while treatment with LiCl for varying times produ that are dorsoanteriorized. This range of phenotypes represents the dorsoanterior index (DAI) (46) indicated by the numb each embryo. In this scale, the normal embryo has a dAi of 5 (in the middle), numbers below 5 represent ventralized ph< numbers above 5 dorsalized embryos. At the extremes, an embryo with a DAI of 0 entirely lacks head and dorsal axial st while an embryo with a DAI of 10 contains only radially symmetrical head structures.
5. Genetics
5.1. Xenopus Genomics
The size of the Xenopus laevis genome has been estimated to be approximately 3*109 bp (37), which is comparable to that of the human genome. The somatic genome has 36 chromosomes (18 pairs). Xenopus laevis is a pseudo- tetraploid organism: evidence for tetraploidy is based on both comparison of DNA content between X. laevis and other Pipidae and the fact that most, but not all, genes examined are duplicated (37). About 20-30% of the entire X. laevis genome consists of repetitive DNA, and more than 2500 genes have been cloned from X. laevis to date and are available in the databases . In addition, the entire 17,553-bp mitochondrial DNA genome of X. laevis has been sequenced and shown to encode 13 proteins, 22 transfer RNA, and two ribosomal RNA (38). There is even a very rough provisional linkage map for X. laevis (37). Finally, the egg contains 4 ng of total maternal RNA, of which approximately 1% (40 pg/egg) is mRNA.
5.2. Genetic Approaches in Xenopus
Despite the fact that there are 40 recessive mutants known in X. laevis (39), its polyploidy (see text above) and large number of pseudogenes cause the system to be not accessible presently to classic genetic analysis. Nevertheless, a large number of approaches have recently made progress toward a genetic analysis of Xenopus embryonic development. Three such approaches will be described: nuclear transplantation, transgenesis, and maternal knock-outs. Recent work targeting Xenopus tropicalis as a bona fide genetic system have been initiated by several groups, however, and classic mutagenesis approaches in progress with this frog should yield their first fruit soon.
5.2.1. Nuclear Transplantation
While nuclear transplantation in mammals, and the generation of the clone Dolly, the lamb have recently received a lot of attention, it is important to remember that the first successful nuclear transplantation was performed in frogs. In 1952, Briggs and King, using the frog Rana pipiens, reported the first successful transfer of nuclei from a blastula to an egg and obtained embryos that survived until neurula stages. Shortly after, two groups using genetic markers demonstrated that cloning could work in X. laevis. It was, however, John Gurdon and colleagues who first obtained a genetically marked, sexually mature cloned frog (40). Nuclear transplantation requires the isolation of nuclei from donor cells and their subsequent placement into an egg in which the natural haploid nucleus has been eliminated. The donor cells can be derived from various tissues at various times in development. In Xenopus, the younger the nuclei, the better the survival rate. Nuclei isolated from blastula or gastrula stages sustain development beyond metamorphosis with higher efficiency than nuclei derived from the skin or intestine of a tadpole. Unlike what has been reported recently in mammals, however, no nuclei from adult frog tissue have been able to carry development beyond metamorphosis. The recipient cell is always an egg in which the haploid genome has been eliminated by UV irradiation. The use of genetic markers in the donor and recipient cells, for example nuclei derived from albino embryos transferred into a wild-type pigmented egg, are used to assay for the successful elimination of the maternal nucleus. After nuclear transfer in this case, an albino frog derived from a pigmented egg represents a successful clone.
A modification of the nuclear transplant approach, in which nuclei from a Xenopus cell line are used for transplantation, has also been reported. This approach provides the advantage of manipulating the genome of the cultured cell prior to implantation. The number of embryos that develop normally after this procedure is low, and they usually do not survive beyond late neurula stages (41).
5.2.2. Transgenesis
Unlike transient expression of RNA or DNA by microinjection, transgenic technology allows stable and regulated gene expression. In Xenopus, unlike mouse, DNA injected into the egg or early blastomeres does not integrate into the genome, for reasons that are not clear. Using a technique originally developed in hydra, Kroll and Amaya reported recently the first successful transgenic frogs (42). This technique, called for restriction-enzyme-mediated integration (REMI), takes advantage of the nuclear transplantation technique in which nuclei are isolated from mature sperm, rather than somatic cells. The tightly packed sperm chromatin is decondensed in vitro and incubated with DNA that has been digested with restriction enzymes. This DNA is incubated with the decondensed sperm nuclei in the presence of a small amount of the same restriction enzyme and DNA Ligase. During this incubation, it is presumed that the restriction enzyme partially digests the sperm DNA; exogenous DNA that is mixed with the genome then integrates at the digested site(s) with the help of the ligase. Although several such transgenic frogs have been made, and this technique is becoming more widely used, no transgenic F1 progeny have been reported yet; specifically, there have been no reports of germline transmission of transgenic DNA. Most of the transgenic Xenopus generated to date do not successfully complete metamorphosis. This is, however, not necessarily a major problem; it is possible to perpetuate a line with nuclear transfer, so there is no requirement for sexual reproduction. Therefore, transgenic lines can, theoretically, be perpetuated indefinitely.
Progress in this field is very rapid, and there are many other attempts in progress. These include the use of transposable elements and retroviruses in various species of Xenopus. It can be safely predicted that generation of transgenic frogs will become routine before the end of this millennium.
5.2.3. Maternal Knockout Strategies
Although conventional knockout or knockin strategies, which are commonly used in the mouse, are not presently available in amphibians, it is possible to eliminate maternal transcripts from the egg selectively to assess their role during early embryogenesis. In this approach, antisense oligonucleotides against specific RNA sequences are microinjected into oocytes (43), which are then reimplanted into a female (44). These eggs are marked so that they can be distinguished from controls, either by using the albino/pigmented technique described above or by incubation with lipophilic vital dyes. Later, these eggs are isolated from the surrogate mother and fertilized in vitro. Their development is compared to controls that are treated in the exact same way but injected with control sense oligonucleotide. A stringent control for the specificity of the phenotype is provided by rescuing the egg after fertilization by injection of sense RNA.
6. Conclusions and Perspectives
In addition to the use of Xenopus laevis embryos and oocytes in molecular studies, there are many other features of Xenopus that make it amenable to molecular studies, in fields as diverse as metamorphosis, tissue regeneration, behavioral studies, physiology, pharmacology, and neurobiology.
Although there is appreciation of the conservation of molecular factors in the vertical evolutionary scheme, it is important not to undermine horizontal differences in strategies of development among different frog species. Frogs certainly provide a fertile terrain to explore these differences at the molecular level, and it would be no surprise if the differences in molecular strategies turn out to be more impressive than the mechanisms that are conserved.