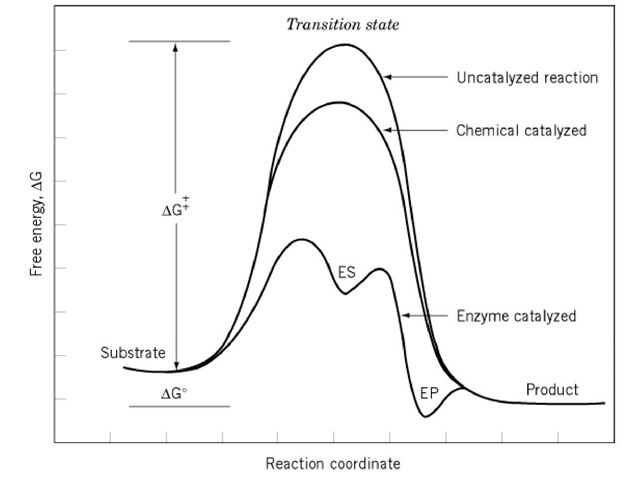
There is no necessary connection between how much and how fast a reaction occurs (see Kinetics). The more stable the products are relative to the starting reactants, the more complete is the reaction. A free energy diagram of the reaction indicates why this is so (Fig. 1). The difference in free energy between the reactant and the product determines the equilibrium constant for the reaction. A large and favorable free energy difference does not, however, ensure that the reaction will occur rapidly. The rate of a reaction is determined by an independent variable, the free energy of the transition state, which is the least stable species occurring during the reaction. If the transition state has a low free energy, the reaction occurs rapidly. The higher the free energy, the slower the reaction. Within the transition state for chemical reactions, covalent bonds are being broken and made.
Figure 1. An example of a reaction coordinate diagram. The reaction coordinate is a measure of the extent to which a chemical reaction has occurred in a molecule. The starting molecule is on the left, the product on the right. The free energy of the molecule is given by the solid line. The species with the highest free energy is the transition state for the reaction. The higher its free energy, the slower the reaction. Chemical catalysis is depicted as occurring because the transition state is stablized. Such stabilization shown for enzymatic catalysis is caused by binding of the reactants to the enzyme.
According to the simplest form of transition-state theory, the rate constant kr for a reaction is defined by the free energy DGJ of the transition state, in the following equation:
where kB is the Boltzmann constant, Tis the absolute temperature, h is Planck’s constant, and R is the gas constant. This equation was derived by assuming that the transition state is in equilibrium with the reactants and that molecules reaching the transition state are converted to products by the most rapid process in which covalent bonds can be broken and made, given by kBT/h; it has a value of
A related concept is the activation energy, which corresponds to the enthalpy of the transition state. Enzymes and other catalysts can be imagined to increase the rates of reactions by lowering the free energy of the transition state, generally by interacting with it and stabilizing it.
Because they are the least stable species during a reaction, transition states are populated for the least amount of time and are not detectable directly. Their natures can be inferred only by studying the effects of systematically varying the conditions of a reaction or the reactants and examining their effects on the rate of the reaction, i.e., the free energy of the transition state (see Free Energy Relationships).