Thioredoxin (Trx) is a 12-kDa redox protein that has a redox-active disulfide bond in its active site with the conserved sequence -Cys-Gly-Pro-Cys- (1, 2). The reduced form of Trx, Trx-(SH)2, is a powerful protein disulfide reductase, and the disulfide in oxidized thioredoxin (Trx^) is reduced by
NADPH and the flavoenzyme thioredoxin reductase (3, 4). Thioredoxin, NADPH, and thioredoxin reductase, the thioredoxin system, exists in all living cells and is a hydrogen donor for synthesizing deoxyribonucleotides by ribonucleotide reductase, which is essential for DNA replication. The thioredoxin system plays a major role in maintaining a reducing environment in the cytosol. Thioredoxin catalyzes dithiol-disulfide oxidoreductions and has a large number of functions (reviewed in Ref. 5), for example, as a hydrogen donor for reductive enzymes, in phage T7 DNA replication, in filamentous phage assembly, in chloroplast photosynthetic enzyme regulation by light, in redox regulation of enzymes and transcription factors by thiol/disulfide redox control, in defense against oxidative stress, and as a secreted cocytokine in mammalian cells.
1. Structure
The three-dimensional protein structures of both oxidized and reduced thioredoxins have been determined in solution by both X-ray crystallography and NMR. They have the same globular "a/b sandwich" structure that is now called the thioredoxin fold. The active-site cysteine residues are located on a protrusion at the C-terminus of a beta-strand and at the beginning of an alpha-helix. The sulfur atom of the N-terminal Cys residue is solvent-exposed and its thiol group is the nucleophile that has a low pKa value in Trx-(SH)2. A unique conserved cis proline residue (Pro76) is adjacent to the active site, which is surrounded by a flat hydrophobic surface used in binding interactions with other proteins. Thioredoxin-fold proteins include a growing superfamily. Some have several thioredoxin domains, as in protein disulfide isomerase (PDI), which catalyzes formation of native disulfides in secreted proteins.
2. Thioredoxin Functions
Thioredoxin was originally defined as a hydrogen donor for Escherichia coli ribonucleotide reductase (1). This enzyme contains a disulfide bond after each catalytic cycle that is reduced by thioredoxin via its general disulfide reductase activity:
The enzymes that reduce sulfate (PAPS reductase) and methionine sulfoxide also use thioredoxin as an electron donor (5). Trx-(SH)2 in E. coli is essential for phage T7 DNA replication in its role as a subunit of T7 DNA polymerase (6). This enzyme is a 1:1 complex of the virally coded polymerase (gene 5 protein of 80 kDa) and the host Trx-(SH)2 (7). The role of the bound Trx is to give the enzyme high processivity in DNA synthesis by forming a complex and does not involve electron transport. Recombinant exonuclease-deficient T7 DNA polymerase is widely used in DNA sequencing. E. coli cells that lack Trx (trxA~) are viable because glutaredoxin and glutathione act as a hydrogen donor for ribonucleotide reductase (8). Thioredoxin, an abundant protein in E. coli, has about 10,000 copies per cell and increases in amount in stationary-phase cells. It is located at the plasma membrane at the junctions of the inner and outer membrane (Bayer’s adhesion sites) (9). This may explain its role in assembly of filamentous phage, where Trx-(SH)2 is essential (10), but Trx-S2 is not. This also gives the phage assembly process a requirement for thioredoxin reductase. The localization of Trx at the plasma membrane in E. coli and its release from cells by osmotic shock is a basis for a Trx-fusion system for overexpressing proteins. Soluble fusion proteins are selectively released from cells by osmotic shock (11). Recently, the gene for a second E. coli thioredoxin, called Trx2 (trxC), has been cloned (12), and those genomes sequenced generally encode two or more thioredoxin genes.
The tryptophan fluorescence of E. coli Trx is strongly quenched by the disulfide bond in Trx-S2 (13). Reduction to Trx-(SH)2 results in three-fold increased fluorescence caused by a localized conformational charge that affects Trp28, whereas the conserved Trp31 still has a low quantum yield (13). The kinetics of oxidation or reduction of thioredoxin are readily measured by fluorescence. Such experiments demonstrated that Trx-S2 is reduced by dithiothreitol (DTT) two orders of magnitude faster than the disulfide bonds of insulin and that Trx-(SH)2 reacts four orders of magnitude more rapidly with the insulin disulfide bonds than DTT (4). This led to the realization that thioredoxin catalyzes the reduction of insulin disulfides by DTT. This can be used as a simple Trx assay by following the turbidity that results from insulin B-chain precipitation (4). The oxidation/reduction potential (Eo’) of Trx-S2/Trx-(SH)2 is -270mV, and a Pro34His mutant has an Eo’ of -235mV (14).
Unique chloroplast thioredoxins in photosynthetic organisms regulate several photosynthetic enzymes by light via electrons from ferredoxin-thioredoxin reductase (15). Trxyregulates fructose- bis-phosphatase and Trxm regulates malate dehydrogenase (16). In each case the enzyme inactive in the dark contains a disulfide bond that is reduced to activate the enzyme in light. Trxh in the cytosol is reduced by NAPH and thioredoxin reductase.
Thioredoxin and thioredoxin reductase from mammals differ in some important respects from the prokaryotic proteins, and they were first purified to homogeneity from rat liver (17) (Table 1). Thioredoxin has roles in regulating many transcription factors by thiol/disulfide redox control that keep critical thiol groups reduced (18). Trx is also a secreted cocytokine from both virally infected and normal cells (19). Secretion occurs by a mechanism that does not require a signal peptide (20). Measurements of human plasma levels of thioredoxin show a correlation with AIDS progression (21). Thioredoxin protects against oxidative stress as an electron donor to thioredoxin peroxidases (peroxiredoxins) (22).
Table 1. Properties of Thioredoxin Systems
|
Organism |
Thioredoxin |
Thioredoxin Reductase |
|
E. coli, yeast, plants |
12 kDa |
Mr 70,000 (2 subunits) |
|
Highly specific |
||
|
Mammalian cells |
12 kDa |
Mr 116,000 (twosubunits) |
|
Inactivated by oxidationof structural SH-groups |
Broad substrate specificity,selenoprotein homologous to glutathione reductase |
3. Sequence Comparisons
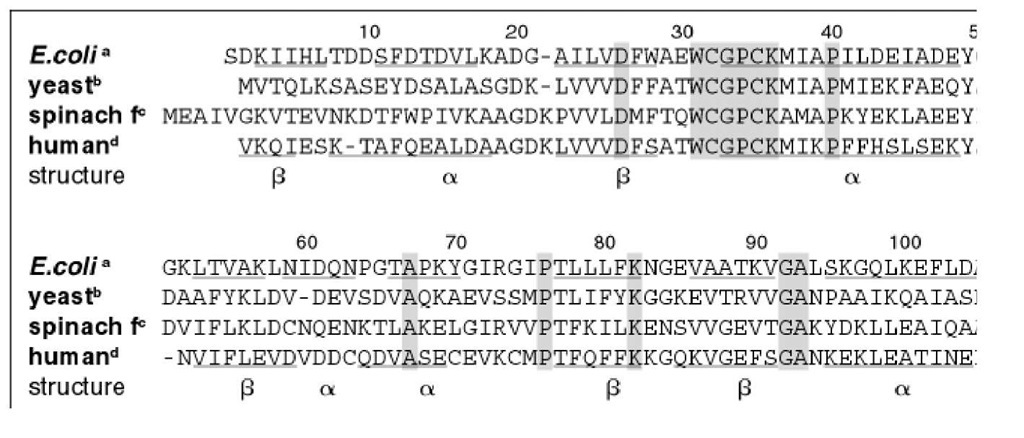
Thioredoxins from archaebacteria to humans have 27 to 69% sequence identity to the well-studied E. coli thioredoxin, which indicates that all thioredoxins have similar three-dimensional structures (23). The amino acid chain length in all organisms studied thus far is approximately the same (see Fig. 1), and has an unusually low frequency of gaps. When gaps exist, they are only one residue long. In addition to the active site sequence Cys32 – Gly33 – Pro34 – Cys35, a number of residue positions are highly conserved (using the E. coli numbering): Asp26, Ala29, Trp31, Asp61, Pro76 (cis), and Gly92. Avian and mammalian thioredoxins contain additional nonredox active cysteine residues that are not involved in redox function but are implicated in regulation. Human thioredoxin forms an inactive intermolecular homodimer via a disulfide bond between Cys73 in each monomer (24). In general, residue substitutions among the thioredoxins occur predominantly on the surface of the molecule and distant from the active site.
Figure 1. Amino acid sequence alignment of selected thioredoxins. Original references to the sequences are given in Re! sequences were aligned on the basis of the three-dimensional structures of E. coli (29) and human thioredoxins (30).
4. Thioredoxin Three-Dimensional Structures
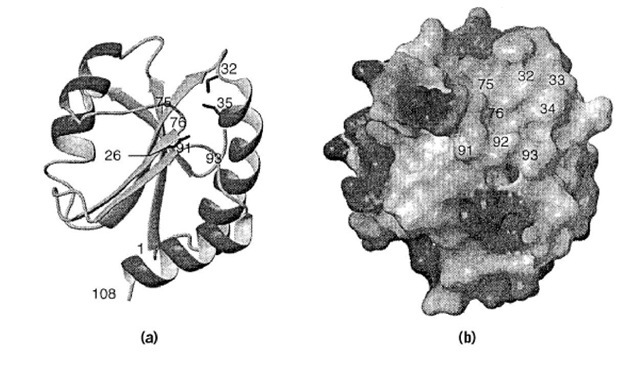
The first three-dimensional structure of a thioredoxin determined by X-ray crystallography techniques (25) was that of the oxidized E. coli protein. It consists of a five b-strands and four a-helices. The molecule is organized into an N-terminal bab and a C-terminal bba motif connected by an_a helix (Fig. 2). E. coli Trx can be cleaved proteolytically into two fragments by trypsin at Arg73, roughly between these two motifs. Although neither fragment alone is active enzymatically, they can reconstitute a native-like molecule that has enzymatic activity (26). The central five-stranded mixed b sheet lies between the second and last helices on one face and the connecting helix on the other.
Figure 2. Representative three-dimensional NMR solution structure of reduced E. coli thioredoxin, illustrating the active site cysteines (Cys32 and Cys35) and the hydrophobic interaction surface area. (a) Cartoon of the polypeptide backbone where the side chains of residues 26, 32, and 35 are displayed as sticks. (b) The molecular surface is colored according to the electrostatic potential (dark gray: positive, medium gray: negative, and light gray: uncharged). Residues are labeled at their approximate positions.
Several high-resolution thioredoxin structures have been determined by using both X-ray crystallography, for example, E. coli (27) and human (28), and NMR spectroscopy, E. coli (29) or human (30). Comparison of the available three-dimensional structures confirms the close structural similarity suggested by their sequence homology. Despite the biochemical evidence of functional differences between the oxidized and reduced forms of the E. coli protein (eg, with T7 gene 5 protein), structural differences between the two forms are subtle and localized to the active-site region (31). Differentiation between the two forms could be the consequence of increased accessibility of conformational states other than the ground state in the reduced form, relative to the oxidized forms. The N-terminal redox-active sulfur atom (Cys32) is located in a protuberance of the molecule and is accessible to solvent from one side in both oxidized and reduced forms of the protein. The pKa of this thiol group is believed to be ~7 which is 1 to 2 pH units lower than normal.
Adjacent to the accessible face of nucleophilic Cys32 is a large, relatively flat hydrophobic patch on the protein surface composed of atoms from residues Gly33, Pro34, Ile75, Pro76, Val91, Gly92, and Ala93 (Fig. 2). The existence of a single hydrophobic interaction surface is consistent with the broad specificity and covalent disulfide intermediate in the mechanism of thioredoxin action (Fig. 3). A number of charged side chains, for example, Glu30, Lys36 and Lys57, are on the opposite face of the protuberance, directly behind the active site cysteine residues, and are of possible mechanistic importance. Largely buried between these residues is the highly conserved Asp 26 which is believed to have an abnormally high pKa of 7.5 (32) and has been proposed as the general base for deprotonation of the C-terminal (Cys 35) cysteine in the mixed-disulfide intermediate.
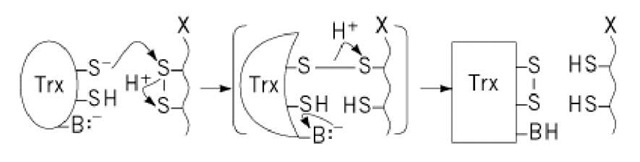
Figure 3. Mechanism of reduction of the disulfide bond of a protein substrate (X) by thioredoxin (Trx). Reduced thioredoxin (left) binds to a target protein via the hydrophobic interaction surface, followed by nucleophilic attack of the N-terminal active site thiolate on the target disulfide in a thiol-disulfide exchange reaction, resulting in a transient protein-protein mixed disulfide (center). Intramolecular attack by the second thiol group of Trx results in oxidized thioredoxin and reduced target protein.
5. Intermolecular Complexes
Structures of intermolecular complexes between thioredoxins and the proteins and fragments with which they interact have helped to elucidate the nature of the protein-protein interactions involved (7, 33, 34). The interface between Trx and its substrate protein includes the aforementioned hydrophobic interaction surface. Interestingly, human Trx binds substrate peptides in two essentially antiparallel orientations, with hallmark hydrogen bonds between the backbone of the substrate Cys residue and backbone atoms centered on the residue of Trx preceding the cis-Pro76. Thus, Trx can utilize the hydrophobic interaction surface to accommodate substrates that have little sequence homology and might also involve molecular chaperone-like conformational changes.
6. Thioredoxin Superfamily
A growing number of proteins contain thioredoxin-like structural motifs, and these proteins are now called members of the thioredoxin superfamily. Proteins that contain the thioredoxin fold can be grouped into at least six classes: thioredoxins, glutaredoxins, DsbA, protein disulfide isomerase, glutathione transferases, and glutathione peroxidases (35). The thioredoxin fold common to each of these protein families consists of the bab and bba motifs, that have insertions or extensions of polypeptide chain. Although the sequence homology among these six classes is limited and no function or activity is common to all of them, there is a functional similarity common to four of these members. Thioredoxins, glutaredoxins, DsbA, and protein disulfide isomerase are redox-active proteins that contain a -Cys-Xj-X2-Cys- active-site motif (where X represents any of the 19 commonly-occurring non-cysteine amino acids). Furthermore, although the remaining two proteins, the glutathione transferases and glutathione peroxidases, lack the -Cys-Xj-X2-Cys- active-site motif, they share with the glutaredoxins a specific interaction with the ubiquitous cysteine-containing peptide glutathione. Interestingly, the orientation of the glutathione in the glutaredoxin and glutathione transferases is similar to that in the cysteine-containing segment of the REF-1 peptide in the complex with human Trx, possibly indicating an overall conserved mode of interaction.