1. Biosynthesis of the Pyrimidine Ring
The biosynthesis of pyrimidine nucleotides is comparable to that of purine nucleotides in several important respects. Both rings are synthesized by de novo pathways that begin with amino acids and their metabolites as precursors. The de novo pathway to pyrimidines is universal among organisms studied thus far, as is the purine pathway. Like purines, pyrimidine bases and nucleosides can be used in "salvage" pathways that involve reutilization of components released in nucleic acid degradation. Salvage capacities vary considerably among organisms and among different cell types in a single organism.
There are also important differences between purines and pyrimidines in the nature of the de novo synthetic pathway. The purine ring is assembled at the nucleotide level whereas, in pyrimidine synthesis, the sugar and phosphate are incorporated near the end of the pathway. Also, purine synthesis is branched, with guanine and adenine nucleotides formed by separate pathways from a common intermediate, inosinic acid. In contrast, pyrimidine ribonucleotide synthesis is unbranched, with uridine nucleotides serving as precursors to cytidine nucleotides.
In bacteria, the principal regulated step, and the committed reaction for the pathway,
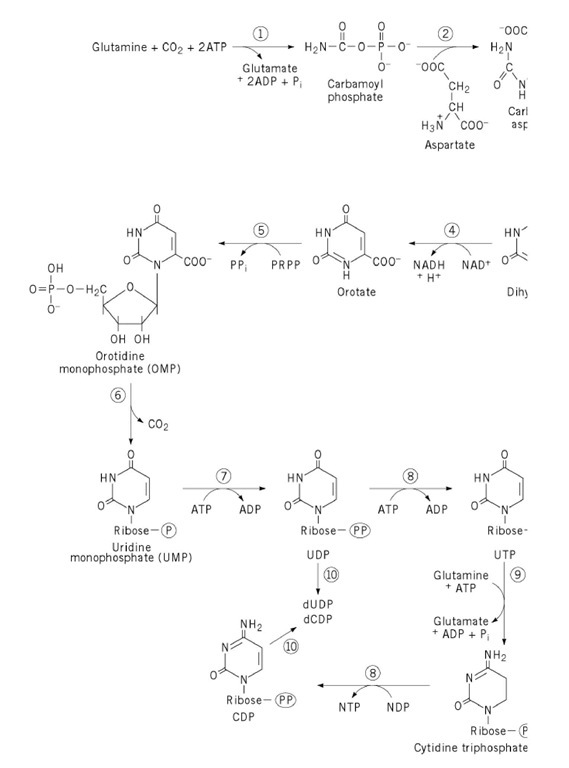
is catalyzed by aspartate transcarbamoylase (ATCase), the enzyme that has perhaps told us more than any other enzyme about allosteric mechanisms in enzyme regulation. The entire pathway is shown in Figure 1 . Control of ATCase involves allosteric inhibition by a pyrimidine end product, CTP, and "feedforward" activation by a purine nucleotide, ATP. The product, carbamoyl aspartate, contains all six of the carbon and nitrogen atoms that will eventually appear in the pyrimidine ring.
Figure 1. De novo biosynthetic pathway to pyrimidine nucleotides. Enzyme names are as follows: 1, carbamoyl phospha 2, aspartate transcarbamoylase; 3, dihydroorotase; 4, dihydroorotate dehydrogenase; 5, orotate phosphoribosyltransferase orotidylate decarboxylase; 7, UMP kinase; 8, nucleoside diphosphate kinase; 9, CTP synthetase; 10, ribonucleoside diph reductase. PRPP is 5-phosphoribosyl-1-pyrophosphate. The circle drawn around the letter P denotes organic phosphate, < triphosphate, as indicated. For the conversion of CTP to CDP by nucleoside diphosphate kinase, the preferred phosphate any, has not been identified.
Carbamoyl phosphate synthetase (CPS), which provides one of the substrates for aspartate transcarbamoylase, is often considered the initial reaction in pyrimidine synthesis. Carbamoyl phosphate is a precursor to both pyrimidines and arginine. Bacterial cells contain a single carbamoyl phosphate synthetase, so that the reaction is not committed to pyrimidine synthesis, and control is exercised at the next reaction, catalyzed by ATCase. Eukaryotic cells, however, contain two carbamoyl phosphate synthetases in different compartments—one in the cytosol, specialized for pyrimidine synthesis, and one in mitochondria, used for the synthesis of arginine (and urea in those organisms possessing a urea cycle for amino acid degradation). Thus, one usually thinks of carbamoyl phosphate synthetase in eukaryotic cells as catalyzing the first step in pyrimidine synthesis (reaction 1 in Fig. 1), with ATCase as reaction 2.
In all cells, carbamoyl aspartate cyclizes (reaction 3) to give a ring compound, dihydroorotate, and this undergoes dehydrogenation (reaction 4) to give the first pyrimidine—orotate, which is 6-carboxyuracil. A phosphoribosyltransferase reaction with 5-phosphoribosyl-1-pyrophosphate (PRPP) gives a pyrimidine nucleotide, orotidine-5′-phosphate (reaction 5). Decarboxylation (reaction 6) yields uridine monophosphate, which undergoes two successive phosphorylations (reactions 7 and 8) to give uridine triphosphate, UTP. UTP is the substrate for an amidotransferase enzyme, CTP synthetase, which transfers the amide nitrogen of glutamine in the ATP-dependent conversion of UTP to CTP (reaction 9).
In most organisms, the synthesis of deoxyribonucleotides occurs at the nucleoside diphosphate level (reactions 10), with reduction of the ribose sugar to deoxyribose in situ (see Ribonucleotide Reductases). UDP is formed as an intermediate in UTP synthesis, whereas CDP is probably synthesized in a nucleoside diphosphate kinase-catalyzed reaction in which CTP serves as a phosphate donor. Because nucleoside diphosphate kinase has very low specificity for both phosphate donor and phosphate acceptor, this represents the most straightforward route for conversion of CTP to CDP. There is a report of a myokinase -type activity in mammalian cells that reversibly transfers phosphate from CTP to CMP, yielding two CDPs (1). If this enzyme plays a major role in CDP synthesis, much of the CMP probably comes from catabolism of cytidine-containing phospholipids, CDP-choline, or CDP-diacylglycerol. CMP is not an intermediate in pyrimidine synthesis de novo.
2. Regulation of Pyrimidine Synthesis
As noted earlier, bacterial ATCase presents a particularly well-understood example of allosteric regulation. Control of pyrimidine ring synthesis occurs also at the level of gene transcription, with an interesting attenuation process (2). Classic attenuation control involves operons of amino acid biosynthesis, where the rate of translation of a messenger RNA determines the folding pattern of a nascent mRNA which, in turn, determines whether the conformation adopted by that mRNA will lead to premature transcriptional termination. In the case of pyrimidine synthesis, the intracellular concentration of UTP inversely controls the transcription of the genespyrB1 andpyrE, which encode subunits of ATCase and orotate phosphoribosyltransferase, respectively; at high concentrations of UTP, the transcription rates are low. Low UTP levels limit the rate of transcriptional chain elongation because the Km of RNA polymerase for this nucleotide is high. Under these conditions, ribosomes translating the nascent mRNA catch up to RNA polymerase, and the interaction interferes with formation of an RNA hairpin loop that acts as a transcription terminator. By contrast, high UTP levels allow RNA polymerase to move fast enough to prevent interference with formation of the transcription terminator.
Another important control mechanism involves allosteric regulation of CTP synthetase . This enzyme is activated by GTP and inhibited by its product, CTP (3). The physiological importance of this regulation is seen in the dramatic effects in mammalian cells of mutations that abolish the feedback inhibition by CTP. Cells bearing these mutations have grossly elevated pools of CTP and dCTP and abnormally small pools of uridine and thymidine nucleotides. The pool imbalance creates a mutator phenotype, and the dTTP deficiency creates a thymidine auxotrophy. Also, a recent report states that phosphorylation of CTP synthetase by protein kinase C decreases the enzyme’s sensitivity to CTP inhibition (4).
3. Multifunctional Enzymes in Eukaryotic Pyrimidine Synthesis
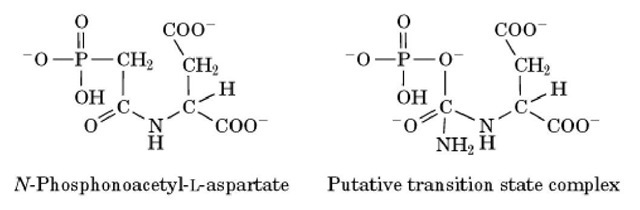
In animal cells, the six enzymes of UMP biosynthesis are encoded by just three genes because of the existence of two multifunctional enzymes. This finding came to light with the synthesis of N-phosphonoacetyl-L-aspartate (PALA), which was produced as a bisubstrate analogue in the ATCase reaction and a potential inhibitor of pyrimidine biosynthesis (Fig. 2). PALA does inhibit pyrimidine synthesis. However, when cell lines resistant to PALA were developed, they were found to have greatly elevated levels not only of ATCase, the target enzyme, but also of carbamoyl phosphate synthetase and dihydroorotase. Analysis showed that the three activities are carried on one 220-kDa polypeptide chain and that three or six chains associate to form one enzyme molecule, called by the acronym the CAD protein (5). Through sequence analysis and limited proteolysis, it has been established that each chain of this multifunctional protein contains an N-terminal carbamoyl phosphate synthetase domain, a C-terminal ATCase domain, and an internal dihydroorotase domain. From the names of these three domains comes the term CAD. The ATCase domain displays significant homology to the catalytic subunit of bacterial ATCase. There is no regulatory subunit counterpart in the eukaryotic enzyme, and control of CAD activities and of the overall pathway is usually exerted at the level of carbamoyl phosphate synthetase.
Figure 2. Structures of N-phosphonoacetyl-l-aspartic acid and the presumed transition state in the aspartate transcarbamoylase reaction.
The fifth and sixth activities in the pathway—orotate phosphoribosyltransferase and OMP decarboxylase—also form a bifunctional enzyme, called UMP synthase. In mammals, these two activities are carried on a single polypeptide chain of about 52 kDa. The enzyme exists as either a monomer or a homodimer, with binding of ligands, particularly orotidine monophosphate, stimulating dimerization (6). Through sequence comparison with organisms containing separate forms of these enzymes, it has been established that the N-terminal part of the UMP synthase polypeptide is the orotidine phosphoribosyltransferase domain; the decarboxylase activity is carried in the C-terminal portion.
What is the biological significance of a protein design that carries either two or three activities in a single polypeptide chain? One likely answer is that it allows for coordinate control of enzymes involved in a single pathway. Another plausible answer is that it permits metabolic channeling—the facilitated transfer of a scarce or unstable metabolite from one active site to the next, so as to drive the overall pathway more efficiently. However, kinetic analysis has not established that either the CAD protein or UMP synthase displays significant channeling activity in vitro. Moreover, the subcellular localization of pathway enzymes makes channeling in vivo seem improbable, at least so far as the overall pathway is concerned. The CAD protein, catalyzing reactions 1, 2, and 3, is located in the cytosol. The product of reaction 3, dihydroorotate, must then move to the outer surface of the inner mitochondrial membrane, where the fourth enzyme, dihydroorotate dehydrogenase, is located. The product, orotate, then moves back to the cytosol for the final two reactions catalyzed by UMP synthase. The necessary migration of an intermediate from one cell compartment to another clearly precludes channeling from steps 3 to 5.
4. Pyrimidine Nucleotide Degradation
Pathways of pyrimidine catabolism are shown in Figure 2. Nucleic acid breakdown usually generates nucleoside 5′-monophosphates, which are cleaved to the respective nucleosides by pyrimidine 5′-nucleotidase. Cytidine is converted to uridine by deamination (the same enzyme also converts deoxycytidine to deoxyuridine). Uridine phosphorylase cleaves uridine, giving uracil plus ribose-1-phosphate. Uracil is reduced to dihydrouracil, which then undergoes a hydrolytic ring opening to give b-ureidopropionate. A further hydrolysis gives b-alanine (a precursor to coenzyme A) plus CO 2 and NH3. A parallel pathway, using mostly the same enzymes, acts on thymine nucleotides. The final product is b-aminoisobutyrate.
5. Disorders of Pyrimidine Metabolism
In contrast to purine metabolic disorders, hereditary deficiencies of pyrimidine metabolic enzymes are extremely rare (6). The most common such deficiency, hereditary orotic aciduria, has been described in just 15 cases. This condition arises from a deficiency of UMP synthase, the bifunctional enzyme that catalyzes reactions 5 and 6 in de novo pyrimidine synthesis. The condition involves excessive excretion of orotate, which usually crystallizes in the urine. A megaloblastic anemia is seen, presumably resulting from insufficient pyrimidine nucleotides to support the nucleic acid synthesis needed for blood cell division and maturation. In addition, some form of mental retardation is seen in orotic aciduria. In all cases tested thus far, the patient responds well to treatment with uridine, which can replete the pyrimidine nucleotide pools through salvage pathways.
Deficiencies of pyrimidine 5′-nucleotidase and dihydropyrimidine dehydrogenase have been described (reactions 1 and 4, respectively, in Fig. 3). The former condition involves a hemolytic anemia; also, the erythrocytes contain abnormally high levels of UTP and CTP. In the latter condition, high levels of uracil and thymine are seen in both blood and urine. Finally, two cases have been described involving deficiency of dihydropyrimidase (reaction 5, Fig. 3), the enzyme that converts dihydrouracil to b-ureidopropionate. The condition involves excessive excretion of dihydrouracil. Because of the rarity of these conditions, few metabolic or clinical details are available.