Zymography is a gel-based enzymatic assay useful for detection and semi-quantitative analysis of proteases and is performed using overlay or co-polymerization techniques (1, 2). In overlay assays, samples are fractionated by SDS-PAGE and, after removal of SDS, the polyacrylamide gel is overlaid on an agarose indicator gel containing protein substrate. Enzyme diffusion into the indicator gel occurs during incubation of the gel sandwich and results in degradation of protein substrate. The indicator gel is then stained to reveal zones of proteolysis, which appear as cleared bands on a dark background. In co-polymerization techniques, protein substrate is incorporated directly into the polyacrylamide gel matrix and, after electrophoresis and SDS removal, gels are incubated floating in buffer (2). In either method, specificity or sensitivity is tailored by modification of acrylamide gel pore size, substrate choice, incubation time, buffer conditions, and by inclusion of activators or inhibitors in gels or buffers.
Overlay assays are advantageous when substrate preference involves proteins that either bind poorly to acrylamide (albumin, casein) or are irreversibly denatured by SDS (collagen type I) and, thus, are not amenable to co-polymerization methods; gelatin, in contrast, is readily incorporated into the polyacrylamide gel matrix, and co-polymerization has been used extensively for assay of gelatinases (2). Overlay methods are also more appropriate for initial quantitative evaluations because enzyme activity is confined within the gel sandwich and, thus, not susceptible to diffusional loss to surrounding buffer, as may occur with co-polymerization assays (1). Furthermore, overlay assays are the method of choice for analysis of complex proteolytic systems in which zymogens, co-substrates, or endogenous inhibitors are relevant, as multiple components can be incorporated into indicator gels.
Representative of a complex proteolytic system is application of the overlay assay to analysis of the plasminogen activator (PA), inhibitor (PAI) system (3, 4). Briefly, samples containing PAs (urokinase [uPA], tissue plasminogen activator [tPA]) and their endogenous inhibitor (PAI) are resolved by SDS-PAGE on 10% gels. Gels are washed with Triton X-100, to remove SDS and allow protein renaturation, and then overlaid on an indicator gel containing plasminogen (zymogen) and fibrin (protein substrate). During incubation, PAs diffuse into the indicator gel, cleave plasminogen to form plasmin, a serine protease that degrades fibrin. After staining of the indicator gel, lytic zones (PA) appear as cleared bands on a dark background (zymography). In contrast, if exogenous PA is also incorporated into the indicator gel, then lysis occurs everywhere except where inhibitor is present; thus, lytic-resistant zones (PAI) appear as dark bands on a clear background (reverse zymography).
1. Materials and Methods
1.1. Materials
Fibrinogen (plasminogen-rich) from Organon Teknika, Holland; SeaKem garose and Gelbond support backing from FMC BioProducts, Rockland, ME; human uPA and tPA from American Diagnostica, Greenwich, CT. All reagents prepared using ultra-pure water.
1.2. Sample Preparation and SDS-PAGE
Samples were prepared, as appropriate, in 2X or 5X Laemmli sample buffer without reducing agent or heating (4). Proteins were resolved by 10% SDS-PAGE (0.75 mm thick slab gels; 20 mA constant current) as previously described (5). After electrophoresis, gels were washed twice (2.5% Triton X-100, 200 mL/gel, 30 min each) to remove SDS and carefully overlaid on indicator gels.
1.3. Preparation of Fibrin Indicator Gel
Plasminogen-rich fibrinogen (5 mg/mL) was solubilized in 0.85% saline (prewarmed to 37°C) with gentle mixing (inversion several times over 1-2 h). Agarose (1%) was separately prepared in PBS (pH 7.4) by heating in a boiling water bath. Dissolved agarose was removed from heat and cooled to 60°C; 15mL of thrombin stock (260 U/mL) and 5 mL fibrinogen solution were then added to the hot agarose (25 mL) in rapid succession and with constant swirling. The agarose-fibrin solution was quickly poured onto the hydrophilic side of a Gelbond support film (~13 x 17cm) using a 25 mL glass pipet and a continuous, vertical motion from left to right; the Galbond support film had been previously stabilized on a glass plate (see below). The agarose indicator gel was allowed to solidify for at least 30 min at room temperature before overlay with the acrylamide gel. Final gel composition was ~0.8% agarose, 0.08% plasminogen-rich fibrinogen, and 0.1 U/mL thrombin (4).
1.4. Fibrin Zymography
Washed polyacrylamide gels were carefully overlaid on fibrin indicator gels and incubated (37°C, 18-20 h) in closed containers containing water-moistened paper towels to provide a humidified atmosphere. After incubation, the acrylamide gel was carefully removed, and the indicator gel was stained (<5min; 0.1% amido black, 70% methanol, 10% acetic acid), destained (70% methanol, 10% acetic acid), and allowed to air dry.
1.5. Reverse Zymography
For detection of PAIs, the indicator gel also contained uPA (0.8 U/mL).
1.6. Notes and Tips
1) Excessive or too vigorous mixing during fibrinogen solubilization results in protein flocculation; 2) volume loss during agarose heating should be reconstituted using ultra-pure water; 3) Galbond has hydrophilic (spreads water) and hydrophobic (beads water) surfaces; water placed in the center of a glass plate adheres the hydrophobic surface of the Gelbond providing a flat stable surface for gel pouring and subsequent handling of gel sandwiches; 4) Work quickly, fibrin polymerization and agarose solidification occur rapidly; delay in pouring produces inhomogeneities within the indicator gel; 5) when disassembling glass plates after electrophoresis, cut the bottom of the acrylamide gel for later orientation; 6) acrylamide gels enlarge and become fragile during washing; handle with care; 7) to position acrylamide gels carefully grasp the upper corners; overlay from bottom to top in a continuous motion to avoid air trapping; 8) avoid repositioning of the polyacrylamide gel when constructing the gel sandwich as artifactual lysis and streaking may result; 9) volumes given are for one full-sized indicator gel; 10) up to two indicator gels can be simultaneously prepared; extra gels can be stored at 4°C for 3-4 days in sealed plastic bags humidified with a damp paper towel.
2. Results and Discussion
Samples containing PAs can be readily analyzed by fibrin zymography using a gel overlay assay (Fig. 1). In such assays PAs and PAIs are resolved by electrophoresis on 10% polyacrylamide gels in the absence of reducing agent or heating. Omission of reducing agent is necessary because SDS may cause artifactual activation of thiol-dependent proteases (i.e., lysosomal thiol cathepsins) as well as inactivation of disulfide-stabilized proteases (i.e., plasminogen activators). Similarly, heating is avoided to preclude artifactual aggregation, activation, or inactivation of susceptible proteases.
Figure 1. Schematic of fibrin overlay assay. (a) Preparation of the fibrin indicator gel. (b) Overlay of the indicator gel (fibrin zymography). For details see MATERIALS AND METHODS section.
During electrophoresis the partially denatured protease migrates with respect to molecular weight. SDS removal after electrophoresis by the nonionic detergent Triton X-100 allows proteins to renature and regain activity. Wash times should be kept to a minimum and accurately monitored to avoid excessive leaching of protease and to assure reproducibility between experiments, respectively.
The gel sandwich should be constructed without repositioning of the acrylamide gel and should be incubated in a humidified atmosphere to avoid gel drying. Incubation times and conditions (temperature, pH) vary depending on the intrinsic properties of the protease being examined as well as its concentration in biological samples. For quantitative purposes, standard curves should be constructed to determine dose-time relationships with respect to size of the lytic zone. Overlay assay of PAs requires incubation for 6-18 h and is temperature-dependent (37°C).
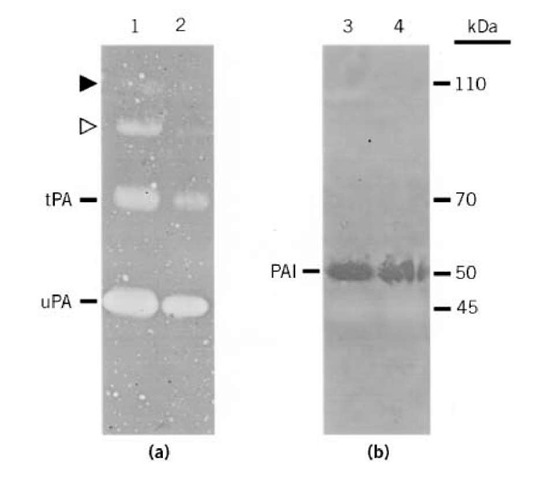
Indicator gels require only brief staining (<5 min) and, after drying, are readily photographed by back lighting. Overlay zymography of PAs demonstrates fibrinolytic zones at molecular weights corresponding to uPA (45 kDa) and tPA (70 kDa) as well as some high (110 kDa) molecular weight bands (Fig. 2A), presumably representing complexes of PA-PAI (3,4). In contrast, reverse zymography demonstrates fibrinolytic-resistant zones at a molecular weight (50 kDa) corresponding to PAI (Fig. 2B). Protease/inhibitor identification can be verified by parallel zymograms in which indicator gels contain PA-specific antibodies or inhibitors i.e., amiloride to inhibit uPA and erythina to inhibit tPA, as has been described elsewhere (4).
Figure 2. Amido black-stained indicator gels. (a) Fibrin zymogram demonstrating uPA (45 kDa), tPA (70 kDa), and higher molecular weight PA complexes (open and closed triangles). (b) Reverse zymogram demonstrating PAI (50 kDa) as well as uPA shadow (45 kDa). Samples 1-4) were from conditioned media of bovine corneal endothelial cells at different growth stages (4).
In summary, overlay zymography is a versatile assay for the detection and semi-quantitative investigation of proteases, which is readily applicable to complex systems. Unique to the overlay method is the opportunity to incorporate into the indicator gel multiple components including activators, inhibitors, antibodies, zymogens, co-factors, and allosteric modifiers. This enables highly sensitive and specific assays to be developed and, thus, affords extensive biochemical investigation of diverse proteolytic systems.