Neutron diffraction and scattering yield structural information on biological molecules and their complexes at multiple levels of resolution. Neutron diffraction and scattering methods generally complement X-ray methods and other structural techniques, frequently offering unique pieces of information that enable one to put together the final jigsaw puzzle picture of how a biological molecule or complex works. Neutron diffraction analysis of crystalline samples is analogous to X-ray crystallography, but at modestly high resolution (<3 A) one can locate precisely individual hydrogen atoms that can be key to enzymatic mechanisms (1) or ligand binding (2). One can also study solvent and its role in protein stability (3-5). Medium-resolution neutron diffraction (3 to 8 A) has been used to orient alpha-helices (6) and to locate specific groups, such as the retinal chromophore in two-dimensional crystals of the membrane protein bacteriorhodopsin (7), and to locate water molecules in DNA structures (8). Low-resolution neutron diffraction (>8 A) with contrast variation gives information on disordered regions in membrane protein crystals, or in large biomolecular complexes such as viruses, and specific examples of this applications are described in Contrast variation. Small-angle scattering of neutrons can yield information on the overall shapes of biological macromolecules in solution and, when combined with contrast variation, can give information on the shapes and dispositions of individual components in biomolecular complexes.
1. Interactions of Neutrons with Matter and Basic Scattering Theory
Neutrons offer a number of advantages as a structural probe in biology. They are less damaging to biomolecules than X-rays. They can be produced in a range of wavelengths from tenths to tens of angstroms and hence are useful for probing many orders of magnitude of dimensions relevant in biological structures: from ~1 to 103 A. Neutrons are neutral particles and interact principally with the atomic nuclei in a sample. Their scattering properties depend upon the complex neutron-nucleus interaction; as a consequence, isotopes of the same element can have very different neutron scattering properties. In addition to the coherent component that can interfere and hence yield structural information the scattering of neutrons by atoms can have a significant incoherent component. The incoherent component is only significant for nonzero spin nuclei (9), and in elastic scattering experiments it gives rise to isotropic scattering that contributes to the background. The incoherent scattering is very large for the hydrogen atom, and consequently for many structural biology applications of elastic neutron scattering it is optimal to minimize the amount of hydrogen in a sample by isotopic substitution with deuterium. Alternatively, investigators have used the incoherent, inelastic scattering from neutrons to probe the dynamics of biomolecules (10). Table 1lists the coherent scattering amplitudes and incoherent scattering cross sections for the nuclei commonly found in biomolecules.
Table 1. Coherent Neutron Scattering Lengths, ^coh, and Incoherent Cross Sections, sinc, for Biologically Relevant Nuclei
|
Atom |
Nucleus |
 |
 |
|
Hydrogen |
 |
80 |
-0.3742 |
|
Deuterium |
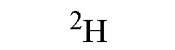 |
2 |
0.6671 |
|
Carbon |
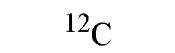 |
0 |
0.6651 |
|
Nitrogen |
 |
~0 |
0.940 |
|
Oxygen |
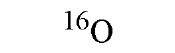 |
0 |
0.5804 |
|
Phosphorus |
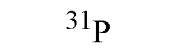 |
~0.3 |
0.517 |
|
Sulfur |
 |
~0 |
0.2847 |
Neutrons can be considered as plane waves with wavelengths (l); when they are scattered by atoms in a molecule, whose positions are designated by the vector r from an arbitrary origin, the resultant interference of the elastic, coherent scattering is related to the spatial distribution of those atoms and can be expressed as
where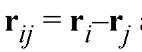 and the summations are over all atoms. Q is the difference between the incident and scattered wave vectors, and its amplitude is 4p(sinq)/l, where 2q is the scattering angle. b is the neutron scattering amplitude for the atom in units of length. Unlike those for X-rays, neutron scattering amplitudes show little or no dependence on scattering angle, because the dimensions of the scattering nuclei are much smaller than the wavelengths of the incident neutrons. In low-resolution solution scattering experiments, one can describe molecules as continuous density distributions, rather than sums of discrete point atom scatterers. Neutron scattering densities are calculated as the sum of the scattering lengths of atoms within a finite volume element, divided by that volume, Sb/V, and a molecule can be described by a scattering density distribution r(r). The total coherent scattering intensity distribution, I(Q), for a randomly oriented molecule in a solvent with a mean scattering density rs is
and the summations are over all atoms. Q is the difference between the incident and scattered wave vectors, and its amplitude is 4p(sinq)/l, where 2q is the scattering angle. b is the neutron scattering amplitude for the atom in units of length. Unlike those for X-rays, neutron scattering amplitudes show little or no dependence on scattering angle, because the dimensions of the scattering nuclei are much smaller than the wavelengths of the incident neutrons. In low-resolution solution scattering experiments, one can describe molecules as continuous density distributions, rather than sums of discrete point atom scatterers. Neutron scattering densities are calculated as the sum of the scattering lengths of atoms within a finite volume element, divided by that volume, Sb/V, and a molecule can be described by a scattering density distribution r(r). The total coherent scattering intensity distribution, I(Q), for a randomly oriented molecule in a solvent with a mean scattering density rs is
The brackets <H> indicate averaging over all orientations of the particle, and the integration is over the volume of the molecule. The term Dr(r) = r(r)-rs is the contrast between the scattering density of the molecule and the solvent, and contrast variation experiments involve the deliberate manipulation of this factor to alter the resultant scattering. The total scattering intensity distribution for a solution of monodisperse molecules will be directly proportional to both the number density and the square of the molecular weight of the particles. Analysis of the small-angle scattering from biological macromolecules in solution yields structural parameters, such as the radius of gyration, R molecular weight, M, and the vector length distribution function, P(r), for the macromolecule. P(r) is sensitive to the symmetry of the scattering particle, and it goes to zero at its maximum linear dimension (see Small-Angle Scattering). P(r) is related to the scattering intensity distribution, I(Q), by a Fourier transformation:
In the case of samples that are ordered in one, two, or three dimensions, the scattered intensity is not spherically averaged, but rather convoluted with the repeating lattice to yield a diffraction pattern that has discrete intensity maxima that can be interpreted in terms of the repeat distances in the sample and sometimes give more detailed structural information about the ordered molecules themselves. For example, one can learn about the superhelical structures formed by protein-DNA complexes (11-13), or about the structures of membranes and the proteins they contain (6, 7). Neutron crystallography is the use of neutron diffraction data from three-dimensional crystals of macromolecules to solve the structure of the crystallized molecule at high resolution. The crystal diffraction data form a complex pattern whose maxima are indexed according to the crystal lattice indices h, k, l. The unit cell diffraction is generally described mathematically in terms of the square of the structure factor Fhkl, which is simply the ratio of the radiation scattering by any real sample to a point scatterer at the origin, and
where x, y, and z are the atomic coordinates of each atom in the crystallized molecule, and the summation is over all atoms. One "solves" a crystal structure by using the measured values to calculate the amplitudes of the structure factors, to which a phase must be assigned in order to extract, by Fourier transformation, the coordinates (x, y, z) for each atom or chemical group in the crystallized structure. The correct assignment of phases (which cannot be measured directly) is known as the "phase problem." Methods for solving the phase problem that are successful for X-ray crystallography, such as molecular isomorphous replacement, are not useful for neutron crystallography. The reason is that they depend upon crystallizing isomorphous molecules with heavy atom labels that scatter X-rays much more strongly than other atoms in the structure and hence can be located and used to generate an initial set of model phases. Neutron crystallography does not have the equivalent of a "heavy atom," because the neutron scattering lengths of most atoms lie in a narrow range of values. As a result, neutron crystallography generally is most useful when the X-ray crystal structure is already available and there are outstanding issues regarding for example, the locations of light atoms. Because neutron scattering amplitudes show no systematic dependence on atomic number, light atoms such as hydrogen are readily located in a neutron crystallography experiment.
One of the largest differences in coherent neutron scattering amplitudes for atoms in biological systems is between the isotopes of hydrogen ( H and H). Note from Table 1that the scattering amplitude for 1H is negative, resulting from a 180° phase shift between neutrons scattered by 1H compared to the other nuclei. Thus, selective deuteration of one component of a complex in solution provides a way of altering the mean neutron scattering density of that component. Furthermore, by changing the deuterium level in the solvent, the neutron scattering contrast of each component is varied. It is these properties that facilitate contrast variation methods that, when combined with small-angle scattering, provide a means for extracting structural information on the individual components of a complex and their relative dispositions in solution. In combination with low-resolution crystallography, contrast variation can be used to locate components or features that are of too low contrast in the corresponding X-ray crystallography experiment to be distinguished.
2. Production of Neutrons
Neutron beams with sufficient intensity for studying the structures of biological molecules require sophisticated technology for their production. There are two classes of sources for high-intensity neutron beams for condensed matter studies operating in the world today: nuclear reactors and accelerators. Nuclear fission reactors produce energetic neutrons in quantities beyond those needed to sustain the chain reaction. The excess neutrons escape and are moderated by various substances surrounding the core of the reactor. The neutrons equilibrated with the moderator substance will have Maxwell-Boltzmann distributions of energies appropriate for the moderator temperature. High-resolution neutron crystallography instruments typically use neutrons moderated at room temperature, yielding a peak in the energy distribution around 1 A. Small-angle neutron scattering benefits from having longer wavelengths available, and thus "cold sources" (eg, liquid hydrogen) are used to slow the neutrons further to have energy peaks in the region 4 to 10 A. Recently, accelerator-based spallation neutron sources (14) have evolved to the point where they can be useful for biological studies. These sources use high-energy proton pulses directed onto a target of heavy nuclei. Neutrons are captured by the heavy nuclei, producing an unstable nucleus that decays, giving off pulses of neutrons in a process referred to as spallation. Pulsed neutron sources are very bright, but on average over time deliver fewer neutrons than a steady-state reactor source. Advantages in pulsed source instrumentation can be gained, however, by using time-of-flight methods to determine the wavelengths of neutrons reaching a detector (15), thus enabling the experimenter to use the entire "white" neutron beam. As spallation sources become more powerful, they are becoming more competitive with reactor sources for scattering applications. In spite of technological advances in neutron sources and instrumentation over the past few decades, however, neutron sources have intensities many orders of magnitude lower than even that of a conventional laboratory X-ray source. Some of this disadvantage can be overcome by long exposures of samples in experiments, because neutrons are less-damaging radiation. However, neutrons are generally used only when their unique properties confer an important advantage that gives specific information that cannot otherwise be obtained.
3. Examples of Neutron Diffraction and Scattering Applications
3.1. High-Resolution Neutron Diffraction Reveals Locations of Hydrogens in Protein Crystals
High-resolution neutron diffraction studies of three-dimensional crystals of proteins have yielded detailed information on the protonation state of individual chemical groups as well as the solvent structure that plays a role in protein folding and stability. In 1980, Kossiakoff and Spencer (1) reported the neutron crystal structure of bovine trypsin covalently inhibited by a transition-state analogue, thus revealing the protonation states of the active-site residues Asp102 and His57 of the catalytic triad. This result resolved the much debated mechanistic issue by showing conclusively that the catalytic base in the transition state of the reaction is His57 and not Asp102. High-resolution neutron diffraction data to 2.1 A from crystals of trypsin (4) localized two-thirds of the waters of hydration expected from thermodynamic data. In comparison, X-ray studies at higher resolution (1.35 A) located about half the waters of hydration, and a number of these were found in the neutron study to be spurious. In another high-resolution neutron diffraction study of carbobon monoxy-myoglobin (5), 87 water and 5 ion molecules were localized, and a number of X-ray-determined waters of hydration were identified as spurious. Neutron crystallography presents a number of difficulties as a result of the relatively low fluxes of neutron sources (compared with X-rays), and the signal-to-noise ratio is low because of the high incoherent backgrounds from hydrogenated proteins. In 1994, Kossiakoff and colleagues (16) reported obtaining 1.5-mm crystals of perdeuterated Staphylococcal nuclease. They showed that there are no structural differences between the protiated and deuterated proteins at 1.9-A resolution. Perdeuteration of proteins is thus a promising tool for gaining signal-to-noise in neutron crystallography experiments.
3.2. Subunit Structure of Biomolecular Assemblies: The Ribosome
Ribosomes are complex assemblies of proteins and RNA that act as protein factories in all living cells. They are composed of a small and large subunit. The 70S ribosome from E. coli is the most extensively studied ribosome, and different subunits have been the target of neutron scattering investigations. Early studies used contrast variation to locate the protein and RNA in both subunits (17-19), and more recent studies have used deuteration, triangulation (20, 22), or triple isotopic substitution (23) methods to give more structural details. In 1987, Moore and co-workers published a complete map of all 21 proteins of the small ribosomal subunit from E. coli using triangulation by measuring distances between pairs of proteins within the small subunit (20). In their experiment, the 30S ribosomal subunit was reconstituted from 16S ribosomal RNA and a mixture of purified 30S proteins, one or more of which was deuterated. Using the small-angle neutron scattering data from these samples, they determined the distance between the centers of mass for each pair of deuterated proteins, plus the radius of gyration for each protein. Over a 10-year period, this group obtained 105 distance data sets relating 93 different protein pairs in the 30S subunit. Using triangulation, they constructed a three-dimensional map of the 21 proteins of the 30S subunit (Fig. 1).
Figure 1. Representation of the neutron map of the 30S subunit of the E. coli ribosome (20). Each protein is represented by a sphere whose volume equals that of the protein. The numbering of the proteins adheres to the standard nomenclature for ribosomal proteins.

![tmp2E-16_thumb[1] tmp2E-16_thumb[1]](http://what-when-how.com/wp-content/uploads/2011/05/tmp2E16_thumb1_thumb.jpg)

