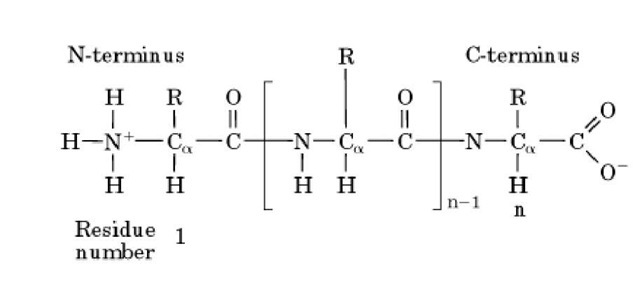
The N-terminus is the term for one end of the polypeptide chain of a protein. Proteins are polymers formed by condensation of the amino groups and carboxyl groups of amino acids. That end of the polypeptide chain having an uncondensed or "free" amino group is called the amino terminus or N-terminus (Fig. 1). By convention, the N-terminus is the first residue in the protein sequence. Similarly, the other end of the polypeptide chain, having an uncondensed or free carboxyl group, is called the carboxyl terminus, or C-terminus, and by convention is the last residue in the protein sequence. The N-terminal amino group is usually positively charged at physiological pH.
Figure 1. Schematic representation of the covalent structure of a protein, showing the N-terminus as the first residue in the polypeptide chain. The square brackets indicate the repeating part of the chain (the backbone), and the R group denotes the variable side chain of each amino acid residue. The C-terminus is the last residue in the chain.
The N-terminal protein residue can be specifically and selectively modified in vivo (see Post-Translational Modifications). Proteins synthesized by the cell, as opposed to chemically synthesized by peptide synthesis, have an initiating methionine residue at the N-terminus. This N-terminal methionine residue is often removed by cellular enzymes. Also, acetylation of the amino group of the N-terminus can be catalyzed by enzymes.
The sequence of a protein can be determined experimentally using a process called the Edman Degradation. This process is based on successive removal and identification of the N-terminal residue of the protein.