Upon infection of a bacterial cell by a bacterial virus, or bacteriophage, the DNA or RNA genome of the bacteriophage enters the cell. The encoded RNA and proteins are synthesized and replication takes place, leading to the production of about 100 progeny phages from each infected cell. Their release is usually accomplished by cell lysis, the breaking open of the cell, which is now dead. This course of events is referred to as a "lytic" infection. For the so-called "temperate" bacteriophages, whose genome invariably consists of double-helical DNA, there is another potential outcome of the infection: The expression and replication of the bacteriophage genome are inhibited, and the DNA gets integrated into the host genome, each DNA strand becoming covalently attached to, and part of, a strand of the bacterial genome. This is the "lysogenic" pathway; the phenomenon is referred to as "lysogeny," the ensuing bacterium is a "lysogen," and the integrated bacteriophage DNA is the "prophage." Such a course of events provides advantages to the bacterium as well as to the bacteriophage. The bacterium becomes immune to subsequent infection by a bacteriophage like the one, whose genome it now harbors. The prophage is provided a "free ride": Its DNA gets replicated with every round of replication of the bacterial DNA. After 8-10 generations, more copies of the bacteriophage genome will have been generated from the original one injected into the cell than if the lytic pathway had been followed. Some bacteriophages have the ability to respond to conditions that threaten the life of their host by killing the host and releasing progeny phages, in a process called "induction." The phenomenon of lysogeny in bacteria has attracted great interest as a model system for viral latency in a eukaryotic cell, when infection results in integration of a virus genome into the cell’s chromosomal DNA, as can occur with herpes or hepatitis virus.
1. Bacteriophage l
Lambda phage (l) is the paradigm of a temperate phage. Not only is the process of establishment of lysogeny better understood for this bacteriophage than for any other, but it is also thought to be representative of the way most other bacteriophages accomplish this feat. Bacteriophage l DNA relies entirely on the bacterial host’s RNA and protein biosynthesis machinery for its expression. It contains all the appropriate signals to enable Escherichia coli RNA polymerase to use its DNA for the synthesis of messenger RNA, and E. coli ribosomes to use these mRNA to program the synthesis of bacteriophage proteins. Soon after the l genomic DNA (Fig. 1A) has been injected into the bacterial cell, an irreversible decision is made, whether the infection will proceed along the lytic or the lysogenic pathways, which are mutually exclusive. Only after the lysogenic state has been well established can viable progeny be obtained by induction (see text below for additional details). In large extent due to the work carried out by Ptashne and co-workers, the establishment of lysogeny in bacteriophage l is understood to a high level of molecular detail (1-4). Here, an outline of the most important steps in the process will be presented.
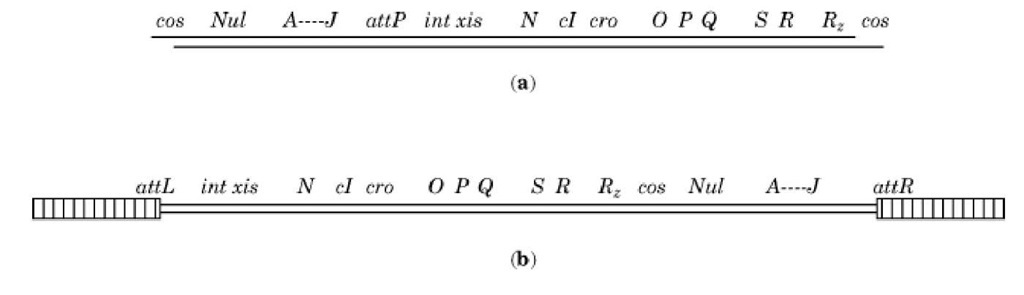
Figure 1. The arrangement of genes on the map of bacteriophage l differs for the DNA in the phage and in the prophage: The two are circularly permuted. The map is not to scale. The double line symbolizes the two strands of the DNA double helix. (a). The linear DNA found in the phage heads (see Lambda Phage). At the ends are the complementary single-stranded regions (cos sites) that enable cyclization. From left to right are: Nu1 (a subunit of terminase), A-J (encoding tail and head proteins), attP (the attachment site), int and xis (involved in integration and excision), N, cl, and cro (regulatory genes discussed here), O and P (DNA replication), Q (antitermination), S and R (cell lysis) and Rz (possibly involved in lysis). (b) Prophage DNA. At the ends are the attL and attR sites, each composed of half of the attB and attP sites originally on the bacterial and phage genomes, respectively (see text). The bacterial DNA is indicated as the striped regions on both sides of the prophage DNA; it, too, is double helical. Note that the cos sites are now internal. The prophage order of genes was obtained by cyclizing the bacteriophage DNA shown in (a) after injection into the cell, then reopening it at the att site in the process of integration into the bacterial genome.
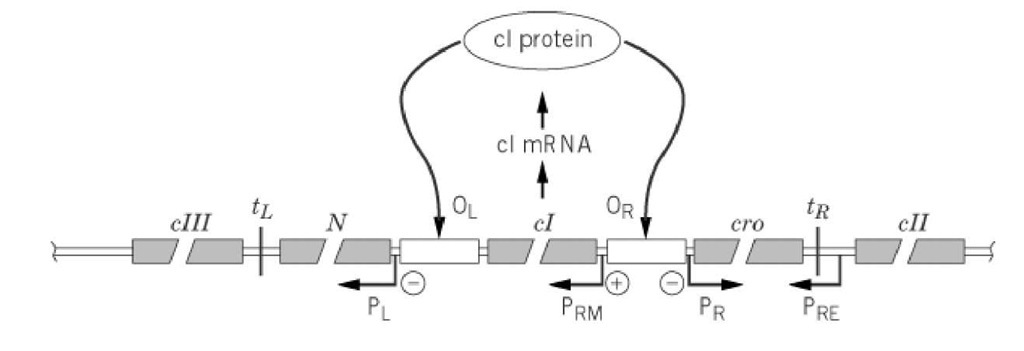
The regulatory region of bacteriophage l DNA is shown in Figure 2; this region represents approximately 10% of the entire l genome (corresponding to the region between xis and O in Fig. 1A). It contains the genes encoding five regulatory proteins [cl, cII, cIII (pronounced "c-one," "c-two," and "c-three," respectively), N, and cro], four promoters where RNA polymerase initiates RNA synthesis (PL, PRM, PR, and P RE), two control regions or "operators" (OL and OR, each divided into three subsites), and two terminators, where RNA synthesis stops and RNA is released (tL and t r). Together these affect the regulation of the life cycle of bacteriophage l.
Figure 2. The control region of bacteriophage l. This map is only intended to show the relative order of important regions and is not to scale. The DNA regions labeled cI, cII, cIII, N, and cro (gray "boxes") are the genes encoding proteins cI, cII, cIII, N, and cro; OL and O R (white boxes) are binding sites for regulatory proteins cI and cro; PL, PR, PRM, and PRE are promoters from which transcription of RNA is initiated in the direction indicated by the horizontal arrows, and tL and t R are terminators where RNA synthesis stops and the transcription complex falls apart. Upward pointing arrows symbolize the synthesis of cI mRNA and protein. The downward arrows point to the binding sites for cI in a lysogen, and they also show the effect of the bound protein on transcription at the nearest promoter (shown as + for activation, – for inhibition). To establish lysogeny, cII binds near the -35 region of PRE to activate transcription from this promoter. Other important interactions and their effects: cro protein binds at OR to inhibit transcription from PRM; N binds to the transcription complex that initiated at PL to prevent termination at t L and to the one that initiated at PR to prevent termination at tR. Note that names of genes are in "italic" letters and those of proteins in "roman" letters.
As discussed (see Lambda Phage), immediately upon entry of the linear l DNA (Fig. 1A) into the cell, it cyclizes by virtue of the fact that its two ends have complementary single-stranded DNA regions, and E. coli RNA polymerase initiates RNA synthesis at P l and Pr, promoters for left- and rightward transcription, respectively (Fig. 2). The DNA sequences of promoters Pre and Prm poorly resemble that of a consensus, or optimal, promoter. Therefore they are not well-utilized by RNA polymerase, unless aided by activator proteins specific to each promoter. Most of the RNAs initiated at Pr and P l are terminated at transcription terminators t l and tR, but they still contain the entire coding sequences for the cro and N proteins, respectively; these two mRNA program E. coli ribosomes to synthesize the cro and N proteins. The cro protein, just like cI and cII a dimeric, sequence-specific DNA-binding protein, represses initiation of RNA synthesis from P rm by binding at OR. The N protein is an antiterminator that enables RNA polymerase to continue beyond tL and t r, allowing additionally the mRNA for cIII to be synthesized from Pl and that for cII from Pr .
These are again translated to give the cIII and cII proteins. At this point, the decision whether to proceed along the lytic or lysogenic pathways is made, in which the cII protein plays a major role: Accumulation of cII protein leads to establishment of lysogeny; otherwise the infection will be lytic. The cII protein activates transcription from Pre (promoter for repressor establishment) (5), leading to the synthesis of cI mRNA, and cI protein. Note that even though the RNA polymerase that initiates RNA synthesis at Pre traverses the cro gene, it does so in the wrong direction to yield functional cro mRNA. Other promoters activated by cII protein are PaQ [involved in the repression of "late" genes (see Lambda Phage)] and P int, from which the mRNA for integrase protein (see text below) is made (6). The cI protein is both a repressor (to which it owes its name lambda repressor ) and an activator of transcription. OL-bound cI protein represses RNA synthesis from Pl, and Or- bound cI represses utilization of the Pr promoter (and synthesis of cro mRNA), by denying RNA polymerase proper access to these promoters. An additional effect of cI bound at Or is to facilitate formation of a functional RNA polymerase-promoter complex at P rm (promoter for repressor maintenance) by favorably contacting PRM-bound RNA polymerase (7). This results in greatly enhanced initiation rates for RNA synthesis from this promoter.
The mRNA synthesized from the Pre promoter provides an initial burst of cI protein (8), needed to jump-start transcription from Prm Once Prm has been activated, the amount of cI mRNA synthesized is sufficient to establish levels of cI protein in the cell sufficient for maintaining both the activated state of Prm and the repressed state of Pr and Pl. The double-helical l DNA is "integrated" into the bacterial DNA with the aid of the integrase protein (encoded by the int gene of l) and a bacterial protein originally discovered by its required participation in this event, integration host factor (IHF). This is a very precise process, always involving the joining of the same regions of bacterial and bacteriophage l DNA, known as attB and attP (attachment sites on the bacterial and phage genomes), respectively. First, attB and attP are positioned next to each other. Next, in a concerted process, both genomes are cut within the attB and attP DNA sequences, and the ends are exchanged and ligated together so that the bacteriophage becomes a prophage, embedded in the bacterial DNA. Because the attP site (Fig. 1A) is different from the overlapping DNA sequences found at the ends of linear l DNA in bacteriophage particles (see Lambda Phage), the map of genes on the prophage is circularly permuted with respect to that of the linear bacteriophage DNA (compare Figs. 1A and 1B). The cI-mediated repression of Pl and Pr effectively silences the integrated bacteriophage l genome, with cI itself the only bacteriophage protein still being synthesized (from mRNA initiated at Prm). This is also the basis for the immunity of the lysogenic cell to subsequent l infections: any invading l DNA will similarly be silenced by cI protein binding to its Ol and O r regions, resulting in repression of transcription initiation from the promoters Pl and P R.
The lytic/lysogenic decision is affected by the growth conditions of the infected cell; if it finds itself in a nutrient-rich environment containing the preferred carbon source for E. coli, glucose, conditions are favorable for the generation of progeny, and the probability is high that the infection will follow the lytic pathway. This is the case for most laboratory media in which bacteria are grown. Conversely, when in nutrient-poor medium, the bacterium will probably be able to support the production of only a limited number of progeny, and mechanisms are in place to increase greatly the probability for establishment of lysogeny. Growth conditions are sensed by a series of events, as outlined in Figure 3, ultimately affecting levels of cII protein in the infected cell (9). The cII protein is highly sensitive to protein degradation by an E. coli proteinase, HflB (or FtsH) (10), which is most active in fast-growing cells. An added twist is that cII is so unstable that, unless it is stabilized by bound cIII protein, it will not accumulate to any appreciable levels even when HflB activity is low. Thus the expression of cIII protein is also required for the establishment of lysogeny. When the bacterium finds itself in nutrient-rich medium, HflB proteinase is active, and cII is rapidly degraded, before it can activate the PRE promoter. Then the promoters PL and P R remain active; any residual activity of P rm will be blocked by cro protein, so no significant cI levels build up, and the lytic pathway will be followed. Conversely, cII levels will be high in poor growth medium, setting in motion the course of events commencing with activation of the Pre promoter to provide the initial burst of cI protein, which favors the lysogenic state. The implementation of the lytic/lysogenic decision occurs at a short region of DNA encompassing promoters Pr and Prm, which has been called a "genetic switch" by Ptashne (3, 4). The molecular details of this switch ensure that it is essentially irreversible: In a lysogen, cI protein not only activates synthesis of its own mRNA from P rm, but it also represses Pr, from which the mRNA for cro, a repressor of Prm, would be made.
Conversely, once the cell has embarked upon the lytic pathway, cro represses the very promoter, Prm, from which mRNA for the synthesis of cI, the repressor of cro mRNA synthesis from Pr, would be initiated.
Figure 3. Summary of the effect of growth conditions on the lytic/lysogenic decision. The bottom two lines of the table, when read from left to right, show two possible courses of events. + indicates high cellular levels of protein; -indicates low levels, or none present.
|
Mediu tn |
Cell growth |
HflB protease |
ell |
cl |
\ development |
|
Nutrient-rich |
Fast |
Active |
— |
— |
Lytic |
|
Nutrient-poor |
Slow |
Inactive |
+ |
+ |
Lysogqnie |
It would not be in the best interest of bacteriophage l to maintain the lysogenic relationship under conditions where the life of its host were in danger. Indeed, reversal of the lysogenic state is initiated when significant damage has been inflicted upon cellular DNA, such as may be the case upon exposure to UV or X-ray irradiation or to chemicals that covalently modify DNA (see DNA damage). Such conditions are much more likely to cause irreparable damage to the DNA of the bacterium than to the integrated bacteriophage DNA, because the former contains 80 times the number of base pairs and presents a correspondingly larger target. As a consequence, the prophage DNA may remain unscathed, even if the host DNA were damaged beyond repair. DNA damage results in the generation of fragments of single-stranded DNA, in the presence of which the bacterial RecA protein facilitates the inactivation of cI protein by a self-inflicted cleavage of its polypeptide chain (11). The resulting relief of repression at Pl and Pr sets into motion a series of events leading to the excision of the bacteriophage DNA from the bacterial genome. This is accomplished by three proteins: In addition to the l integrase and bacterial IHF proteins, which were responsible for the integration reaction, the excisionase protein, encoded by the l xis gene, is also required. Further development proceeds along a lytic-like pathway (see Lambda phage), eventually leading to cell death and the release of progeny phages.
2. Other Temperate Bacteriophages
Several so-called lambdoid phages, with extensive sequence homology to bacteriophage l, have been characterized that behave similarly to phage l in the establishment of lysogeny. The genomes of many other bacteriophages, with less homology to bacteriophage l DNA, also integrate site-specifically into the bacterial genome, but a notable exception is the E. coli bacteriophage "mu." Just like phage l, bacteriophage mu is able to subvert the E. coli synthetic machinery to express and replicate its own genomic DNA. Again, there is mutually exclusive expression of the mu repressor, c (the equivalent of the phage l cI protein), which silences the genome, and a protein specific for the lytic pathway, ner (which plays a role analogous to that of cro in bacteriophage l). However, the factors determining whether one or the other will predominate are not yet fully understood. Bacteriophage mu is unique among temperate bacteriophages, in that integration of its DNA into the bacterial genome is an integral part of both its lytic and its lysogenic modes of development. The initial integration is conservative, that is, the actual DNA molecule that entered the cell becomes part of the E. coli genome, similar to integration of bacteriophage l DNA. Integration always involves the attachment of the same regions of the mu genome (near the ends of the linear bacteriophage mu DNA injected into E. coli) to the bacterial DNA, but in contrast to bacteriophage l, the sites on the E. coli genome where integration takes place are almost entirely random. It is the distinct ability to integrate almost anywhere in the bacterial DNA that has given mu its name: In lysogenic strains, the integrated mu DNA can disrupt various genes of the bacterium and thus inactivate them. Bacteriophage mu has been used as a mutator agent to generate mutants useful for genetic studies.
Bacteriophage mu also stands out in that the divergence between the lytic and lysogenic pathways takes place subsequent to the integration event. If the integrated bacteriophage DNA is silenced by the c repressor, the cell becomes lysogenic. On the other hand, the ner protein may predominate, preventing accumulation of c protein; then phage expression and replication may occur to initiate lytic development. In this case, replicative integration takes place, that is, copies of the mu genome integrate at different sites on the bacterial genome, in steadily increasing numbers. Eventually they will be excised (ie, cut out of the bacterial DNA), packaged into bacteriophage particles, and released upon cell lysis and death. Mu integration has attracted considerable interest because, in this respect, mu DNA behaves similarly to a class of mobile genetic elements called "transposons."
In addition to E. coli, a large number of bacterial species have been found to harbor prophages, and thus to be lysogenic for the corresponding bacteriophages. Examples include pathogenic as well as nonpathogenic bacteria, gram positive as well as gram negative, such as Salmonella, Staphylococcus aureus, and various Streptococci and Mycobacteria. With few exceptions (eg, P22 phage, a l-like bacteriophage of Salmonella ), however, the details of the relationship between these bacterial hosts and their bacteriophages are yet to be determined. Finally, some phages establish a relationship with their host that resembles lysogeny, in that the cell is not lysed. This is the case for the filamentous bacteriophages of E. coli (eg, M13 phage), whose genomes are single-stranded DNA. Subsequent to infection, progeny virus is continuously produced and released, without cell lysis, from the infected cells, which keep dividing. There is, however, no repression of genes of the bacteriophage genomic DNA, nor does this DNA integrate into the host genome, and the infected cells are not considered to be lysogens.