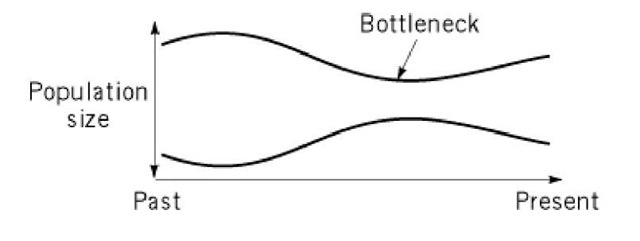
Figure 2. Bottleneck effect due to a transient decrease in the population size.
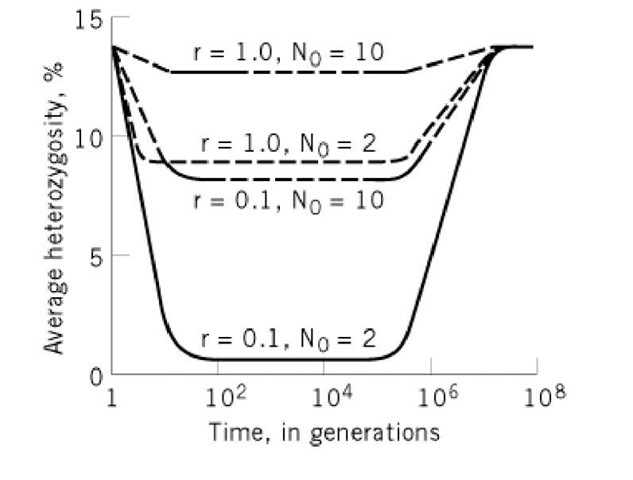
Figure 3. Decrease in the heterozygosity of individuals of a population resulting from the bottleneck effect. The bottleneck size is given by N0. The intrinsic growth rate is given by r. Following the bottleneck, it is assumed that the population grows uniformly (2).
The four-helix bundle is a common protein motif that is observed in protein structures, in which four a-helices pack together lengthwise, in an antiparallel manner (Fig. 1). The four a-helices that form the bundle can derive from four independent polypeptide chains or from a single polypeptide chain. This structural motif has been observed both as an isolated three-dimensional fold and as a domain within much larger and more complex protein structures. The helices of four-helix bundles are longer than average (~20 residues, as opposed to the usual 10) and are amphipathic, with hydrophobic residues buried in the core. The antiparallel packing of the helices in the bundle may be favored by interaction between the helix dipoles.
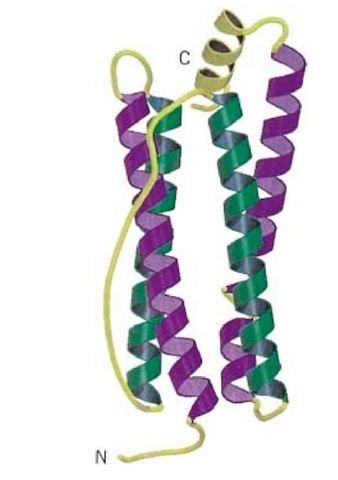
Figure 1. Schematic representation of the backbone of a four-helix bundle protein, taken from the structure of bullfrog ferritin (1). This structure has the up-down-down-up topology; the two "up" helices are shown as purple coils and the two "down" helices are shown as green coils. Note the long connecting loop between the second and third helix. Connecting loops and an additional C-terminal helix are shown in yellow. The N- and C-termini are labeled. This figure was generated using Molscript (2) and Raster3D (3, 4).
The four-helix bundle is one of the simplest protein folds and is used by a wide range of proteins with diverse functions. Proteins with a four-helix bundle structure include growth hormones, cytokines, and ferritins. The four helices may splay apart to generate a binding pocket for cofactors and metal ions, or they may coil around each other to form a supercoil. In the latter case, the twist of the helices in the coiled coils alters the usual helical structure so that there are 3.5 residues per turn, rather than 3.6. This means that every seventh residue in the helix is identically placed with respect to the axis of the helix. The seven positions of this heptad repeat are denoted a to g, and residues a and d point into the core of the bundle and are usually hydrophobic.
Several classes of four-helix bundle motif have been defined. These include (a) the simple up-down-up-down topology, where consecutive helices have short connections and alternating directions, (b) the up-down-down-up topology, where there is a long loop or crossover connection between helices 2 and 3, and (c) the up-up-down-down topology that is typical of the cytokine four-helix bundles, with two long crossover connections between helices 1 and 2 and helices 3 and 4. In most cases, the helices are aligned so that each pairwise interaction is antiparallel. There are also sub-classes within each of these classifications. The simplicity of the four-helix bundle motif has made it a common target for protein design.