A chromosomal domain can be defined at either a structural level or a functional level. At this time, there is frustratingly little information concerning the relationship between these two organizational levels. Our most thorough understanding of chromosomal organization is for the most condensed, and hence most visible, of chromosomes, those at metaphase . Although folding of DNA into nucleosomes leads to a sevenfold compaction in length, and the subsequent folding of arrays of nucleosomes into the chromatin fiber to a further sevenfold compaction, a massive 250-fold compaction of DNA follows the organization of the chromatin fiber into a metaphase chromosome. One model proposed to account for this compaction suggests an organization of the fiber into loops that are radially arranged along the axis of the chromosome (1). It has also been suggested that such loops represent functional chromosomal domains.
The evidence for the organization of the chromatin fiber into loops attached to a central axis in normal cells comes from several experimental approaches. Long-standing observations on the morphology of lampbrush chromosomes in amphibian oocytes show a succession of loops emerging from a single chromosomal axis. Worcel developed a model for the E. coli chromosome that predicted its organization into independent domains or loops. Then these studies were extended to interphase chromosomes from Drosophila cells. Intact chromosomes were subjected to very mild digestion with DNase I to produce single-strand nicks. Then the resulting chromosomal fragments were examined in sedimentation velocity centrifugation experiments to determine their size. It was found that the length of the fragments decreases gradually until a plateau value of approximately 85,000 bp of DNA is reached (2). This suggests that the chromatin fiber is organized into fairly uniform domains containing about 100 kbp of DNA complexed with protein. A second approach to determinating the size of structural domains in the chromosome involves electron microscopic examination of nuclei extracted from histones by exposure to high-salt solution. It is possible to measure the length of DNA directly on the microscope grid from where it exits a residual nuclear structure (the nuclear matrix or scaffold) to where it reenters this structure. Estimated loop sizes between 40 and 90 kbp were obtained using this technique, which were consistent with biochemical measurements (3).
The development of pulsed-field gel electrophoresis allowed more systematic analysis of the separation of cleavage sites following mild nuclease digestion of nuclei (4). This technique allows resolving very large DNA molecules by agarose electrophoresis. Nuclei of eukaryotic cells contain an endonuclease (which was first responsible for the discovery of the nucleosomal repeat) that can be activated under controlled conditions (by the addition of exogenous and which is believed to have little sequence specificity. It might be expected that cleavage of DNA by this enzyme would be inhibited by folding of DNA into the chromatin fiber, but that structural discontinuities, perhaps where the loop is attached to the chromosomal axis, might allow cleavage if the enzyme were activated. Activation of this enzyme followed by resolving the resultant DNA fragments on pulsed-field gels would potentially allow determining loop sizes. A related approach to this problem uses the observation that topoisomerase II is a major component of chromosomes and, potentially, of the nuclear matrix or scaffold. The enzymatic action of topoisomerase II introduces a double-strand break into DNA, which is then resealed. These double-strand breaks can be stabilized by the use of specific drugs (e.g., epipodophyllotoxins) that inhibit the enzyme (5). Preferential cleavage sites in nuclei spaced 50 to 300 kbp apart were detected using both the endogenous nuclease or topoisomerase II in the presence of the specific inhibitors of the rejoining of the double helix. Closer analysis revealed a hierarchy of digestion, where the 300-kbp cleavage products appeared before those of 50 kbp. It has been suggested that the 50-kbp intermediate in digestion represents the first level of organization of the chromatin fiber into loops, whereas the 300-kbp kinetic intermediate represents the next level of organization. Taken together, these observations establish a strong case that large independent loops (50 to 100 kb) of the chromatin fiber represent a unit of chromosomal structure.
and which is believed to have little sequence specificity. It might be expected that cleavage of DNA by this enzyme would be inhibited by folding of DNA into the chromatin fiber, but that structural discontinuities, perhaps where the loop is attached to the chromosomal axis, might allow cleavage if the enzyme were activated. Activation of this enzyme followed by resolving the resultant DNA fragments on pulsed-field gels would potentially allow determining loop sizes. A related approach to this problem uses the observation that topoisomerase II is a major component of chromosomes and, potentially, of the nuclear matrix or scaffold. The enzymatic action of topoisomerase II introduces a double-strand break into DNA, which is then resealed. These double-strand breaks can be stabilized by the use of specific drugs (e.g., epipodophyllotoxins) that inhibit the enzyme (5). Preferential cleavage sites in nuclei spaced 50 to 300 kbp apart were detected using both the endogenous nuclease or topoisomerase II in the presence of the specific inhibitors of the rejoining of the double helix. Closer analysis revealed a hierarchy of digestion, where the 300-kbp cleavage products appeared before those of 50 kbp. It has been suggested that the 50-kbp intermediate in digestion represents the first level of organization of the chromatin fiber into loops, whereas the 300-kbp kinetic intermediate represents the next level of organization. Taken together, these observations establish a strong case that large independent loops (50 to 100 kb) of the chromatin fiber represent a unit of chromosomal structure.
Many studies have focused on the non-histone proteins in the nucleus that might represent sites of attachment of chromatin at the base of loops and the DNA sequences associated with them. The biochemical nature of the nuclear skeleton, the nuclear scaffold, and the nuclear matrix that might organize DNA in the nucleus has been the subject of much debate. Initially, the metaphase scaffold of a chromosome had a morphological definition as the complex structure at the axis of a mitotic chromosome, visualized after swelling and extraction of the histones. Biochemical extraction with high-salt (2 M NaCl) or with the detergentlike lithium diiodosalicylate (LIS) was used to define the residual nucleoprotein complex at which DNA was attached to the chromosome during interphase. Now this nuclear "matrix" (after high-salt extraction) or "scaffold" (after LIS extraction) is known to contain a substantially more complex group of proteins than the metaphase scaffold itself (6). The use of these nonphysiological extraction procedures was criticized because they cause rearrangements of protein-DNA interactions and nonspecific aggregation. An alternate strategy to study nuclear infrastructure is to encapsulate cells in agarose, to extract most of the chromatin, and to leave the nucleoprotein complexes essential for nuclear integrity under physiological conditions. This last methodology generates a nuclear "skeleton" that can transcribe and replicate DNA,which indicates that functionally relevant enzymatic complexes are retained. Recent comparative experiments confirm that the nuclear matrix or scaffold interacts with gene-poor regions of the genome, whereas the nuclear skeleton interacts with gene-rich regions (7). It is now clear that the function and composition of the nuclear skeleton is very different from that of the nuclear matrix or scaffold. All define chromosomal domains, yet the reasons for generating attachments to the chromatin fiber differ.
The nuclear "scaffold" is the best-defined entity with respect to both biochemistry and genetics. A major set of proteins found in the scaffold fraction includes Sc (scaffold proteins) I, II, and III (8). The function of Sc III is unknown. Sc I (170 kDa), however, is now known to be topoisomerase II, and Sc II is known to be a heterodimeric coiled-coil protein. The enzymatic activity of topoisomerase II passes DNA strands through one another. It can cause a double strand break in DNA and rejoin it. Antibodies to topoisomerase II allowed demonstrating that the protein is an integral component of mitotic chromosomes (9). Moreover, the efficiency of recovery of total cellular topoisomerase II in the scaffold fraction (>70%) makes it unlikely that the association with the scaffold fraction is accidental. Consistent with the current view that the chromatin fiber folds further into the chromosome, immunolocalization data show that topoisomerase II is found in a large number of discrete foci scattered throughout the axial region of chromosomes. These foci are very uniform in size, suggesting that they represent discrete structural complexes. Each is believed to be an anchoring complex to which chromatin loops are attached. The presence of topoisomerase II in these complexes can be rationalized by the necessity of unraveling DNA knots and tangles that are inevitably generated during processive enzymatic processes, such as DNA replication and transcription . In fact, if topoisomerase II is inactivated in vivo by mutation, the mutant cells die because they cannot separate their chromosomes at the end of mitosis (10).
The Sc II protein has a much more active role in directing mitotic chromosomal condensation than topo II. Now Scll is recognized as a member of the stability and maintenance of chromosomes (SMC) family of proteins. The function of this family of proteins was first characterized in the yeast, Saccharomyces cerevisiae (11). SMC proteins are conserved in structure from fungi to vertebrates. Each consists of five major regions: a nucleotide-binding region, a region of the alpha-helix with the potential to form a coiled-coil protein-protein interaction domain, a hinge region, a second coiled-coil domain, and a carboxy-terminal region. Mutational analysis indicates that all of these domains are required for SMC protein function. SMC proteins assemble into oligomeric structures. There are at least four different SMC proteins in S. cerevisiae, and all are essential for viability, which suggests that they have essential nonoverlapping functions. The phenotypes of SMC mutant cells in yeast fail to undergo mitosis, and the undivided nucleus splits partially. This phenotype is very similar to that of topoisomerase II mutants in yeast. Closer investigation reveals that SMC mutant cells fail to condense and segregate their chromosomes (11). In spite of this clear genetic evidence that topoisomerase II and the Sc II proteins have essential roles in chromosomal organization, evidence that they have a specific association with DNA is lacking thus far. Thus the nucleic acid contribution to the assembly and function of chromatin loops or structural domain remains unclear.
It has been suggested that the nuclear matrix or scaffold contains specific DNA sequences known as scaffold or matrix attachment regions (SARs and MARs). Evidence that the sequences function in chromosomal dynamics comes from the synthesis of an artificial protein that preferentially binds to these AT-rich sequences (12). This protein interfered with the chromosomal dynamics normally observed during nuclear decondensation or chromosomal condensation in Xenopus egg extracts. In the absence of the identification of bona fide scaffold attachment sequence-binding proteins in vivo and examination of their function, however, whether scaffold or matrix attachments have an active role in chromosomal function remains speculative. Such attachments might promote transcriptional activity in vivo (13). In plants there is compelling evidence that AT-rich segments of the genome that have been biochemically defined as scaffold attachment regions promote the activity of genes in cis . Histone H1 binds preferentially to these AT-rich DNA sequences. It has been suggested that proteins, such as high mobility group (HMG I/Y) might displace histone H1 selectively from scaffold attachment regions that contribute to the local control of transcriptional activity (14). It must be proven that this type of selective association of proteins with scaffold attachment regions occurs in vivo . It has long been known that lysine-rich proteins like histone H1 interact with AT-rich DNA. Hence the significance of in vitro binding experiments that enrich lysine-rich proteins bound to AT-rich scaffold attachment regions remains questionable. A subfamily of sites determined by such procedures as matrix attachment sites (A elements) can be found at the boundaries of a 24-kbp region of DNase I-sensitive chromatin containing the chicken lysozyme gene. Sippel and colleagues demonstrated that the A elements insulate a gene from chromosomal effects in stable transformants, but they are not required in transient assays for high levels of gene activity. These A elements also give high-level position-independent, copy-number dependent expression of a trans gene in transgenic mice (15).
Evidence for the functional specialization of chromosomal domains came initially from cytological analysis of polytene chromosomes. Immunologic staining has revealed the specific distribution of several non-histone proteins, including RNA polymerase II and proteins apparently responsible for generating inactive heterochromatin (HP1, see Chromocenter). Grossbach and colleagues used antibodies against variants of histone H1 to demonstrate that the localization of particular linker histones is highly specific for individual chromosomal domains within polytene chromosomes (16). In contrast, a similar approach with antibodies raised against HMG14-related proteins led to the general immunofluorescent staining of transcriptionally active domains. Turner found that individual chromosomal domains within polytene chromosomes can be significantly enriched with forms of histone H4 that have particular states of posttranslational modification (17). This is where a covalent modification such as acetylation of lysine residues is added by an enzyme after the protein has been synthesized. These experiments indicate that specific proteins and modifications can be targeted to particular chromosomal regions.
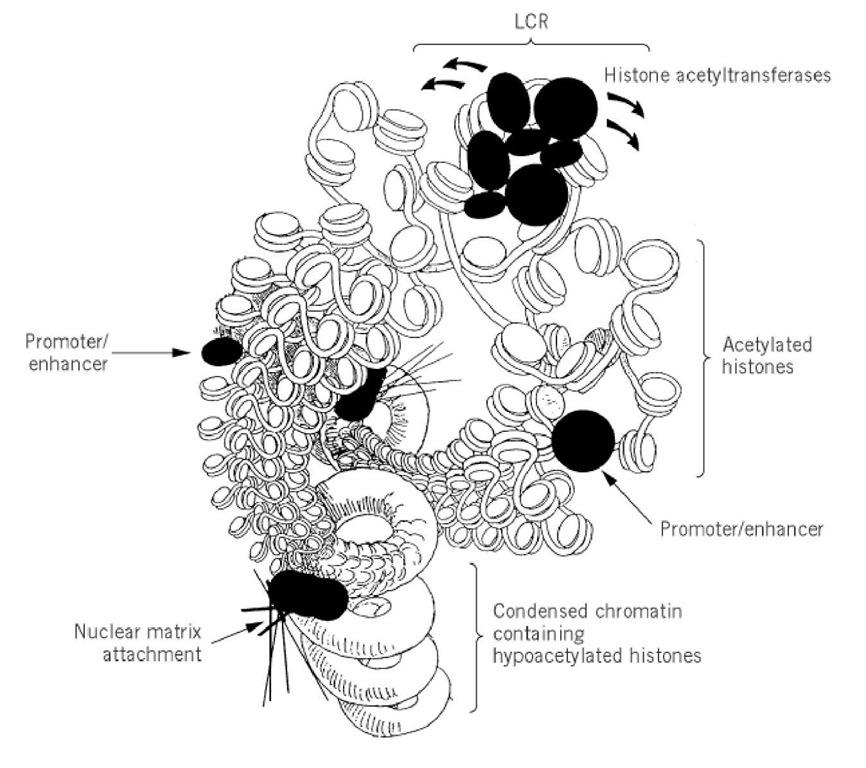
Additional support for the division of the chromosome into individual functional domains came from the discovery of locus control regions (LCR). DNase I hypersensitive sites are useful in defining important DNA sequences. DNase I cleavage of the human b-globin locus revealed the existence of four strongly nuclease-sensitive sites located 10 to 20 kbp upstream of the cluster of human b-globin genes (18) (see Contiguous Genes). These sites, known as locus control regions (LCRs), represent cis-acting elements that allow genes integrated into a chromosome to be expressed independently of chromosomal position, i.e., position effects are abolished. Consequently, LCRs allow each copy of a gene integrated in multiple copies to be expressed equivalently within a particular chromosomal domain, so that gene expression is copy-number dependent. When all four DNase I hypersensitive sites comprising the LCR are placed adjacent to reporter genes, they function like enhancers. However, three of the four sites do not function as enhancers in transient expression sites but only do so after incorporation into the chromosome. This suggests that the LCRs may play a special role in stabilizing an accessible chromatin structure distinct from the function of a normal enhancer element (19) (Fig. 1). Several models have been proposed for LCR function, including unraveling of the chromatin fiber, functions similar to normal enhancers, and stabilization of specific nucleoprotein complexes that are functional for transcription only in the presence of the LCR. Evidence consistent with the propagation of an altered chromatin structure comes from transgenic experiments in which LCRs confer hypersensitive sites and general DNase I sensitivity, whereas promoter elements do not. This suggests that LCRs may be able to overcome the inhibitory influences of localized regions of heterochromatin. It has been suggested that LCRs or regions adjacent to them might define the boundaries of an active chromatin domain through attachment to a nuclear matrix or scaffold. At this time there is no positive evidence to suggest that this is the case in vivo.
Figure 1. A model for locus control region (LCR) function where the LCR defines a functional chromosomal domain. We propose that the LCR recruits histone acetyltransferases that acetylate histones in the functional domain and lead to an expanded and destabilized chromatin fiber. Within this domain are promoter and enhancer sequences that themselves function more effectively when chromatin higher order structure is destabilized. In this model nuclear matrix attachment sites are boundaries to separate the LCR-controlled chromosome domain from condensed chromatin containing hypoacetylated histones.
Schedl and colleagues have used transposable elements (P-elements) that can introduce stable transformants into the germ line of Drosophila melanogaster to define a distinct "insulator" element that might help delimit functional chromosomal domains. Using the hsp70 gene, these investigators defined DNA sequence elements that contain DNase I hypersensitive sites to either side of the hsp70 genes. These specialized chromatin structural (scs) elements conferred position-independent, copy-number-dependent transcription from the white promoter, with the exception of one insertion into heterochromatin within the Drosophila X-chromosome. Importantly, the scs elements do not behave as scaffold attachment regions that establish a functional separation between the two sequences (20). It has been recognized that the Drosophila suppressor of hairy-wing protein and the complex that it assembles with the gypsy transposable element have comparable "insulating" properties.
It is important to observe that scs, gypsy, or A elements block enhancer function if the element is between the enhancer and the promoter. This capacity to block activation or silencing effects has led to the current definition of these elements as insulators. Enhancers normally act over very long distances (many kilobases). It is hard to imagine how a short DNA sequence (100 to 200 bp) could inhibit DNA looping, which suggests that enhancers in this case function by an alternate mechanism, perhaps related to chromatin folding or nuclear compartmentalization (see Figure 1). Additional insight comes from experiments suggesting that gene regulation occurs by progressively altering the structure of chromatin domains that are continuous in the chromosome. In the bithorax complex, three genes ( Ubx, abd-A and Abd-B ), are aligned 5′ to 3′ in the order in which they are expressed during development and, remarkably, in the structures whose origin they control in an anterior to posterior direction in the fly. It has been suggested that each of these three large (> 20 kbp) segments of DNA are activated in succession. Several mutations in the bithorax complex are consistent with this hypothesis because by removing a boundary between two domains of chromatin, the anterior-posterior boundary between different structures is also removed. In this model, boundary elements prevent the propagation of a particular chromatin modification by functioning as a barrier. Once again, this type of genetic analysis provides strong evidence for the existence of chromosomal domain boundaries.