2. Diversity of Aspartate Transcarbamoylase
2.1. Prokaryotes
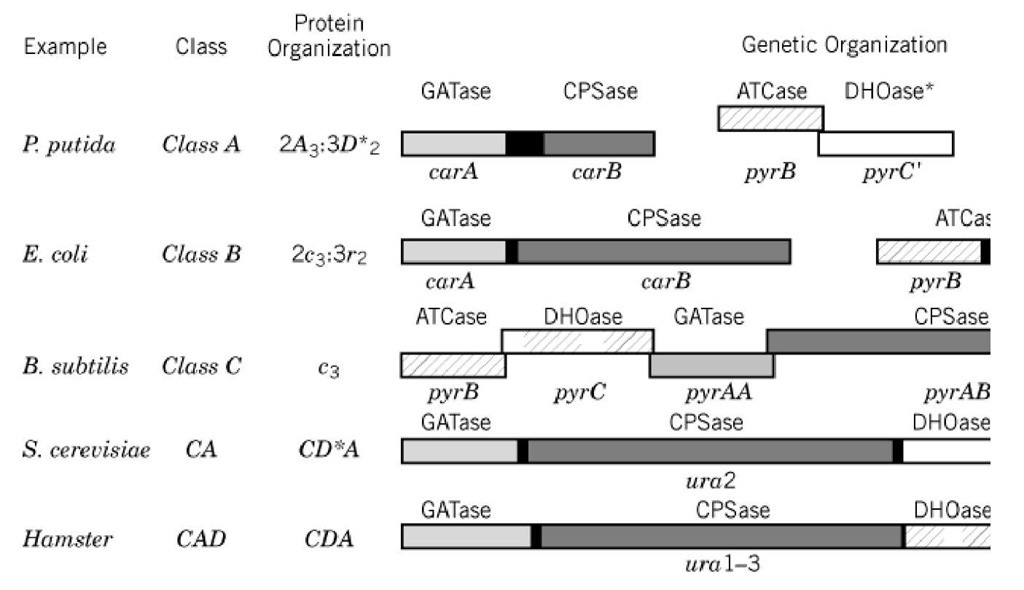
With the exception of some anaerobic protozoan parasites, all examined organisms are capable of de and have been found to possess the enzyme aspartate transcarbamoylase. The earliest classification o and Jones in 1969 (16), based on enzyme size and response to nucleotides, partitioned the enzyme in ATCases are the largest ATCases found in bacteria and were initially described for the pseudomonad ATCases of E. coli and other members of the Enterobacteriaceae; whereas (3) class C is the class wi until recently was restricted to Gram-positive bacteria such as Bacillus (Fig. 5). The class A ATCas in size, and the intact enzyme exists as two catalytic trimers of 35 kDa in dodecameric association w chains (2A3:3D2). In P. putida, this 45-kDa chain has been shown to be a nonfunctional homologue the third enzyme in de novo pyrimidine biosynthesis immediately following ATCase in the biosynthe.
Figure 5. Organization of the first three enzymes and genes of pyrimidine biosynthesis: glutamine amidotransferase (G DHOase. Although multiple examples are known for each category, the examples selected to represent each class are fr subtilis (13), S. cerevisiae (9), and hamster (14). The genetic organization is represented according to the following: Bo enzyme designations above and gene designations below. Noncoding DNA that joins genes transcribed into polycistron segments. Overlapping genes are represented by overlapping boxes. Although all seven cistrons of pyr metabolism are c organization of only the first three is shown here.
The Bacillus subtilis enzyme at 100 kDa is a typical class C enzyme and corresponds in size, architei catalytic trimer of the E. coli enzyme. Class C enzymes are composed of three identical polypeptide 34 kDa each and have no associations with other enzymes in the pathway. Furthermore, these enzym regulatory subunit nor the attendant allosteric regulation. As a consequence of the active site being s1 catalytic chains, the class C homotrimer may be considered the catalytic core of the bacterial ATCas pyr genes are scattered around the chromosome and are not coordinately regulated, all seven B. subti the same operon and transcribed on a single messenger RNA. The 3′ ends of the reading frames over downstream open reading frames for all cistrons in the cluster except pyrB and the preceding ORF1. repressed by pyrimidines, and expression of aspartate transcarbamoylase has been shown to decline i sporulation. The mechanisms for the nutritional and developmental regulation of pyr gene expressio been elucidated.
Although the class B enzymes appeared to be restricted to the Enterobacteriaceae, recent studies sug (7) may also contain very similar c6:r6 enzymes. The E. coli ATCase provides the textbook example inhibition by pathway end-products CTP and UTP, and activation by the end-product of the parallel ATP. However, the various ATCases from different tribes of the Enterobacteriaceae display diverge inhibition or activation. For example, while CTP serves as an allosteric feedback inhibitor in the E. c effect or serve as an allosteric activator in other Enterobacteriaceae (Table 3). Some of these diverge the basic regulatory paradigm of inhibition by pathway end-products as a mechanism to conserve eni of unneeded products. Nonetheless, the discovery of CTP + UTP synergistic inhibition provided a co the allosterically regulated enzymes: Since the concentration of ATP in actively growing cells is 2 to continually activated by ATP unless CTP or CTP + UTP are present to reduce the activated enzyme in which CTP is an activator, the combination of CTP and UTP always serves as an effective antagoi:
Table 3. Classification and Allosteric Characteristics of Bacterial Aspartate Trai
|
ATCase Class Characteristics |
Bacterial Species® |
Alloster |
|
ATCase A |
Pseudomonas fluorescens |
No activation UMP Inhibition |
|
Acintobacter calcoaceticus |
||
|
Azomonas agilis |
||
|
Azotobacter vinelandii |
||
|
ATCase B1 (I)b |
Escherichia coli |
ATP activation |
|
Salmonella typhimurium |
CTP, CTP + UTP |
|
|
ATCase B2 (IV) |
Yersinia intermedia |
ATP activation |
|
CTP, UTP inhibiti |
||
|
ATCase B3 (V) |
Erwinia carnegiana |
No activation |
|
Erwinia herbicola |
sCTPc, CTP + UT |
|
|
ATCase B4 (IV) |
Yersinia entercolitica |
ATP activation |
|
No inhibition |
||
|
ATCase B5 (IV) |
Yersinia kristensenii |
No activation |
|
Yersinia frederiksenii |
No inhibition |
|
|
ATCase B6 (II) |
Aeromonas hydrophila |
CTP, ATP activati |
|
Serratia marcescens |
CTP + UTP antag |
|
|
ATCase B7 (III) |
Proteus vulgaris |
CTP, ATP activati |
|
CTP + UTP Inhibi |
||
|
ATCase C |
Bacillus subtilis |
None |
|
Streptococcus faecalis |
||
|
Staphylococcus epidermidis |
a Class A and C examples are from (15).
b Tribal classifications (given in parentheses) are according to Bergey’s Manual of Determinative Ba< subgroups of the ATCase class B enzymes. c sCTP indicates only slight inhibition by CTP (>20%).
Due to the oligomeric nature of the class B enzymes, it has been possible to form hybrids by combin enzyme with the regulatory subunits of different enzymes that have diverged allosteric patterns. The: have demonstrated that the regulatory dimer determines the nature of the allosteric control of ATCas chimeric proteins have been constructed by intragenic fusion of the CP domain with the Asp domain Alio domain) forming several novel protein structures. In one instance, this type of protein engineeri chimeric enzyme by fusion of the domains of the hamster ATCase cDNA and the bacterial pyrB gen active ATCase trimers that, although unstable, could marginally satisfy physiological requirements.
Table 4. The Regulatory Chain of Class B ATCases Dictates the Allosteric
|
Source of subunit |
Response to Effectors® |
||
|
Catalytic Subunit Regulatory Subunit |
ATP |
CTP |
|
|
E. coli |
E. coli |
+ |
- |
|
S. marcescens |
+ |
+ |
|
|
P. vulgaris |
+ |
+ |
|
|
S. marcescens |
E. coli |
+ |
- |
|
S. marcescens |
+ |
+ |
|
|
P. vulgaris |
+ |
+ |
|
|
P. vulgaris |
E. coli |
+ |
- |
|
S. marcescens |
+ |
+ |
|
|
P. vulgaris |
+ |
+ |
|
a + = activation; – = inhibition.
2.2. Eukaryotes
The ability to form chimeric proteins opens the possibility that intragenic fusions could provide a me development of new proteins by the fusion of domain modules. Among the biosynthetic pathways of examples of single polypeptides that carry multiple enzymatic activities. Eukaryotic ATCases provic examples of a multienzymatic protein. In lower eukaryotes, such as yeast, the first enzyme in the pat synthetase (CPSase), and ATCase are physically linked, forming the CD A protein fusion complex. cerevisiae and S. pombe, the enzyme architecture includes four domains; three functional domains co amidotransferase (GLNase), CPSase, ATCase activities, and one dihydrorotase-like (D*; DHOase) c the third enzymatic activity of the pyrimidine biosynthetic pathway just following ATCase. This stru the architecture previously discussed for the class A prokaryotic enzymes, which possess a domain w DHOase. The regulation of this complex includes the feedback inhibition of both CPSase and ATCa though the carbamoylphosphate produced by GLNase/CPSase is tightly channeled to ATCase and a the GLNase/CPSase alone should be sufficient to regulate pyrimidine metabolism.
In higher eukaryotes, the CA complex is associated with a functional DHOase domain producing a m (physically arranged in a CDA sequence). This complex provides the central metabolic control for py CPSase subjected to allosteric inhibition by UTP and activation by 5-phosphoribosyl 1-pyrophospha regulatory and catalytic functions involve a single polypeptide chain, the multifunctional complex m structure. A series of proteolytic and genetic truncation studies over the last 15 years have provided structure of CAD is well-defined and simple: Each enzymatic domain is separated from the others by polypeptide linker, and each domain can function in the absence of the other activities.
There are a number of proposals regarding the evolutionary role of the multienzymatic architecture. I channeling occurs between CPSase and ATCase of the bifunctional CD A complex. Channeling of s successive enzymatic activities are carried out on the same protein complex and could conceivably p advantage by limiting the loss of intermediate products. However, this is not always the case, as the t freely releases the products of CPSase and ATCase, whereas ATCase and DHOase can readily utiliz outside the enzymatic complex. In the case of CAD and similar enzymatic complexes, coordinate ge alternative regulatory advantage for the evolution and maintenance of large enzymatic complexes. T complexes would provide for a smaller number of independent genes to regulate and simplify the co’ subcellular localization of multiple enzymatic activities.
In summary, the de novo biosynthetic pathway involves the set of reactions that supplies UMP from metabolic pathways: aspartate, glutamine, ATP, PRPP and carbon dioxide. The de novo pyrimidine ] enzymatic steps from carbamoyl phosphate synthetase (CPSase) to orotidylate decarboxylase (OMP evolution has developed a variety of regulatory controls and genetic organizations. Independent of it ATCase provides an important regulatory component of de novo pyrimidine biosynthesis in all free-l maintaining homeostasis in intracellular nucleotide pools is an essential consideration for metabolism enzymological regulation mechanisms are critical for balancing the nucleotide precursors of DNA/R.