The amino acid arginine is incorporated into the nascent polypeptide chain during protein biosynthesis in response to six codons—CGU, CGC, CGA, CGG, AGA, and AGG—and represents approximately 5.7% of the residues of the proteins that have been characterized. The arginyl residue incorporated has a mass of 156.19 Da, a van der Waals volume of 148 A3, and an accessible surface area of 241 A2. Arg residues have average conservation during divergent evolution; they are interchanged most frequently in homologous proteins with lysine, the other basic residue.
The Arg side chain consists of three nonpolar methylene groups and the strongly basic d-guanido group:
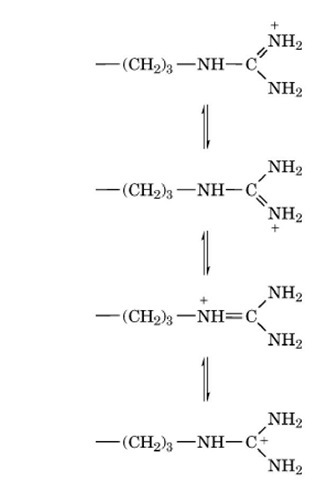
With a p value usually of about 12, the guanido group is ionized over the entire pH range in which proteins exist naturally. The ionized guanido group is planar as a result of resonance:
and the positive charge is effectively distributed over the entire group. In the protonated form, the guanido group is unreactive, and only very small fractions of the nonionized form are present at physiological pH values. The guanido groups of Arg residues are almost invariably at the surfaces of native protein structures, and virtually no Arg residues are fully buried, but the nonpolar part of the side chain, and the adjoining polypeptide backbone, are frequently buried within the interior. Arg residues favor the alpha-helical conformation in model peptides and also occur most frequently in that secondary structure in folded protein structures.
Proteinases frequently cleave polypeptide chains adjacent to Arg residues, as in the processing of pro-hormones, such as pro-insulin, at pairs of basic residues.
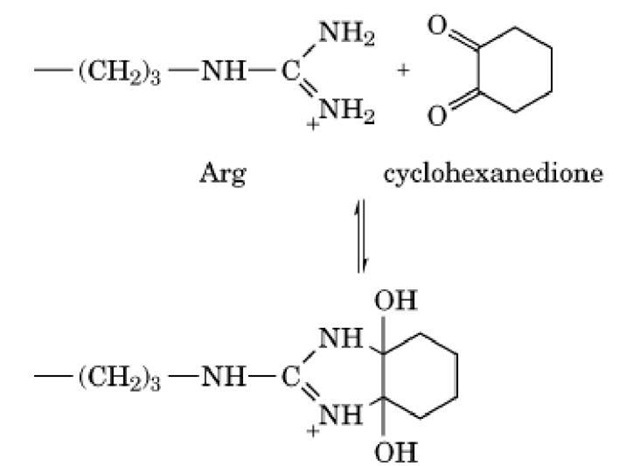
The guanido group can form heterocyclic condensation products with 1,2- and 1,3-dicarbonyl compounds, such as phenylglyoxal, 2,3-butanedione, and 1,2-cyclohexanedione:
These reactions occur readily because the distance between the two carbonyl groups of the reagents closely matches that between the two unsubstituted nitrogen atoms of the guanido group. The adduct formed can be stabilized further by the presence of borate, which complexes with the adjacent hydroxyl groups.
The guanido group can be cleaved by hydrazine (H2NNH2) to produce the side chain of ornithine:
This reaction is, however, often accompanied by cleavage of the polypeptide backbone.