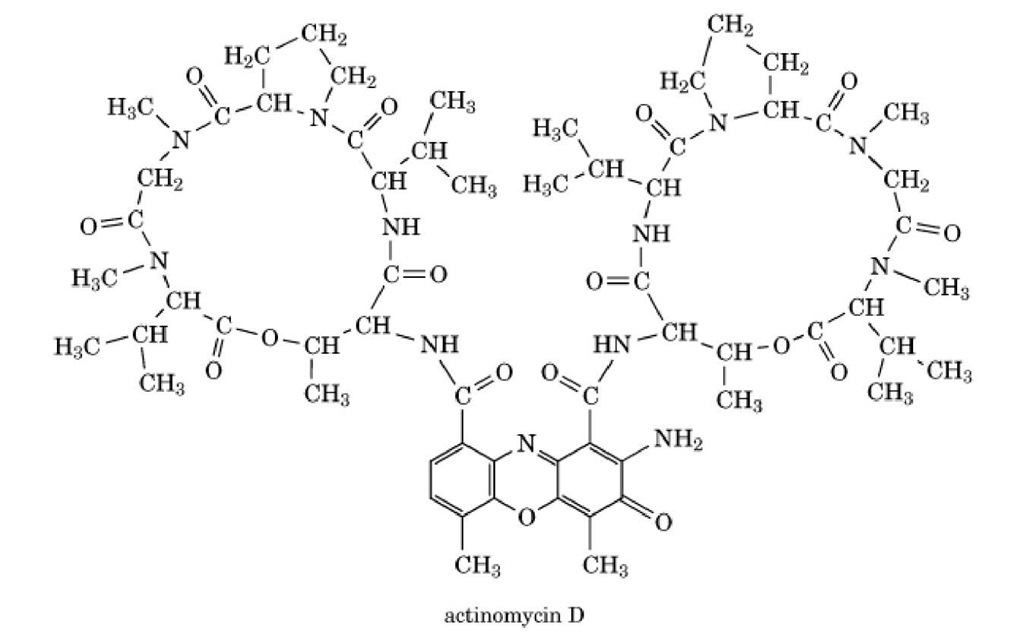
Actinomycins are chromopeptide antibiotics produced by various species of streptomycetes (1). They differ only in their content of certain amino acids in their peptide rings (Fig. 1). Actinomycin D, which is active against several forms of cancer, has the distinction of being the first antibiotic discovered (in 1943) to possess useful antitumor activity. It is still used in the clinic for that purpose. It occupies a unique place in the origins of molecular biology for quite a different reason, however, namely its astounding specificity as an inhibitor of DNA-dependent RNA synthesis, which enabled it to play a vital part in the discovery of messenger RNA and helped uncover the early evidence that transcriptional regulation of gene expression is fundamental to cell growth and development (2).
The result of its near-absolute specificity for binding tightly to double helical B-form DNA is a total blockade of transcription by RNA polymerase, leading to cessation of synthesis of all forms of RNA, stable as well as unstable. Elongation of growing RNA chains is promptly terminated; there is no particular effect on the process of initiation of transcription. Thus actinomycin was used in the 1960s to measure the kinetics of breakdown of pulse-labeled RNA, thought to reflect at least partly the turnover of mRNA, and to establish the requirement for new RNA transcription in such processes as induced enzyme synthesis, mechanisms of steroid hormone and peptide hormone action, and early embryonic development. Replication of DNA viruses is usually strongly inhibited by actinomycin, but many RNA viruses are completely insensitive to the antibiotic; this is clear evidence that their life cycles do not require the participation of DNA at any point. Because actinomycin acts essentially identically to block transcription in all cells, once it has gained access to the nucleus, it is equally applicable to the study of gene activity in eukaryotes as well as prokaryotes (it is generally ineffective against gram-negative bacteria simply because of a permeability problem, for if the walls of such cells are digested away, or otherwise weakened, the protoplast is revealed as fully sensitive to the antibiotic).
Figure 1. The structures of the actinomycin antibiotics.
Actinomycin does not bind to RNA or to single-stranded DNA, and its affinity for double-helical DNA is related to the latter’s content of guanine nucleotides; synthetic polynucleotides composed entirely of A • T base pairs do not interact with the antibiotic at all. It took some time to establish the nature of the actinomycin-DNA complex, and for many years the matter was controversial. Eventually the issue was resolved in favor of intercalation as a result of careful structure-activity comparisons (1), binding measurements with a wide variety of synthetic as well as naturally occurring DNAs (3, 4), hydrodynamic experiments with circular DNA (5), X-ray crystallography (6), and high-resolution nuclear magnetic resonance (NMR) (7). A landmark was the determination of the structure of a crystalline 1:2 actinomycin:deoxyguanosine complex, which revealed for the first time that the antibiotic has an axis of near-perfect twofold rotational symmetry, which allows it to react with two guanine nucleosides in a symmetry-related fashion (6). This observation immediately suggested plausible intercalation of the phenoxazinone chromophore between two G • C base pairs of DNA at a rotationally symmetrical site centered around a![]() step. The strong preference of actinomycin for such sites in DNA was elegantly confirmed by Footprinting experiments a decade later (8, 9). With its tricyclic aromatic chromophore firmly embedded between the base pairs, the cyclic pentapeptide rings of the antibiotic are left neatly filling the minor groove of the distorted B-form helix, where they form numerous additional van der Waals contacts that help to stabilize the complex; positioned this way, their intrinsic right-hand twisted disposition with respect to the intercalated chromophore makes perfect sense, and the whole antibiotic molecule occupies a site covering about six base pairs in the minor groove.
step. The strong preference of actinomycin for such sites in DNA was elegantly confirmed by Footprinting experiments a decade later (8, 9). With its tricyclic aromatic chromophore firmly embedded between the base pairs, the cyclic pentapeptide rings of the antibiotic are left neatly filling the minor groove of the distorted B-form helix, where they form numerous additional van der Waals contacts that help to stabilize the complex; positioned this way, their intrinsic right-hand twisted disposition with respect to the intercalated chromophore makes perfect sense, and the whole antibiotic molecule occupies a site covering about six base pairs in the minor groove.
Detailed kinetic studies have revealed that both the association and dissociation reactions are complicated and require several rate constants to fit the data (3, 10). The slowest processes are characterized by time constants in the range of minutes, conspicuously slower than those measured for most other DNA-binding drugs, and there is a correlation between the slowest rate constant for the dissociation reaction and the efficiency of inhibition of chain elongation by RNA polymerase. This has led to the notion that reversibly bound actinomycin molecules serve as relatively long-lived blocks to the progression of the transcribing enzyme along its template, providing a partial explanation for the extreme effectiveness of actinomycin as an inhibitor of RNA synthesis. What is not so obvious is why the functioning of DNA as a template in replication should remain unaffected even at much higher concentrations of actinomycin (2).
The critical determinant that enables the antibiotic to recognize its preferred binding sites in DNA seems to be the 2-amino group of the guanine nucleotide, as is also true for numerous other ligands (11). If the 2-amino group is removed from guanines (leaving inosine-cytosine base pairs) and transferred to adenines (forming 2,6-diaminopurine-thymine base pairs), the sites to which actinomycin binds are relocated accordingly (11, 12). The molecular recognition process includes the formation of hydrogen bonds between the purine 2-amino groups and the carbonyl substituents of the L-threonine residues in the peptide rings of the antibiotic; there are also hydrogen bonds from the same threonine residues to the N(3) atoms of the purine nucleotides at the binding site (6, 12). Some of the kinetic complexity of the association/dissociation reactions no doubt reflects a "shuffling" process, whereby actinomycin initially interacts with a variety of potential binding sites on the DNA lattice, then migrates one-dimensionally to locate its preferred binding sites marked by a pair of purine 2-amino groups suitably disposed in the minor groove of the double helix (13).