1. The Actin Microfilament System
Actin was identified more than 50 years ago as an essential element of the force generating apparatus of muscle cells (1, 2). Together with myosin, tropomyosin, and a large number of other proteins, actin filaments constitute sarcomeres, which are ordered serially along contractile myofibrils (3, 4). In the mid 1960s, actin (5) and myosin (6, 7) were recognized as major structural components of the eukaryotic cytoplasm (8-10); for an early review of the field see (11). The unexpected observation that actin was the inhibitor of DNase I (12), and that the DNase inhibitor could be crystallized (13), led to the identification of the actin monomer-binding protein, profilin (14). The properties of profilin seemed to explain how unpolymerized actin could exist at high concentrations in cells without spontaneously polymerizing (15). It is now known that there are several other proteins controlling actin polymerization, and today the family of actin-binding proteins contains over 100 members including proteins that sequester actin monomers (profilins, thymosins), sever (gelsolins), depolymerize (cofilins), and cross-link (villin, fimbrin) actin filaments. Further characterization of the nonmuscle myosins (16), and an increased appreciation of the role of polymerization of actin suggests how cells can extend pseudopods and drag themselves along extracellular matrices (17, 18), and exhibit the high degree of motility seen on the surface of lymphocytes and in malignant cancer cells. This entry will describe general characteristics of actin-binding proteins, the analysis of which in many cases has reached the atomic level.
Actin is an obligatory component of all eukaryotes, and is one of the most highly conserved proteins during evolution. Its sequence has varied less than 10% during the last 1200 million years (19). In mammalian cells, six different isoforms are known, of which the muscle specific a-isoform and the nonmuscle b-isoform are the most studied [for review, see (20)]. On the basis of structural homology an actin-related protein, FtsA, which is involved in cell division, has been recognized in prokaryotes (21). Also, in eukaryotes, there are several actin-related proteins, ARPs (22).
Actin can be kept in a monomeric form only under low salt conditions or in the presence of actin sequestering proteins (review 20). Under physiological salt conditions, actin polymerizes into long filaments. In this form, actin participates in force generation and motile activity. The actin filament is polar, with a rapidly growing (+)-end (barbed end) and a slow growing (-)-end (pointed end). In the cell, actin filaments grow by addition of monomers at the (+)-end, which is located at the outer edge of cell surface protrusions: lamellipodia and filopodia. In the submembraneous zone all round the cell, actin filaments are organized into sheets and bundles held together along their lengths by a variety of crosslinking proteins, which differ in length and flexural rigidity.
The myosins vary in structure; some are globular in shape, like myosin I, and do not polymerize, whereas others have a long a-helical, supercoiled, rod domain and can form filamentous superstructures in the cytoplasm (16). Together, actin and myosin transduce chemical free energy of adenosine trisphosphate (ATP) to mechanical work, not just in muscle cells, but in all eukaryotic cells. In different states of organization, the actomyosin system, is responsible for muscle contraction, cell locomotion, and cell division, and for many different local cellular transport processes (4). In nonmuscle cells, the actomyosin system is referred to as the microfilament system.
It is based on the organization of actin filaments, whose formation, organization and activity depend on the activity of a large number of actin-binding proteins, including myosin.
Microfilaments are present in particularly high concentrations in close apposition to the inner leaflet of the lipid bilayer (23-27), where they are directly or indirectly linked to cell surface proteins (receptors and adhesion proteins) continuously probing the extracellular environment. The lipid bilayer of the plasma membrane has been likened with "light machine oil" (28). It is an excellent electrochemical barrier, but it is the dense cortical weave of microfilaments which confers mechanical stability and deformability to the cell surface. Thus, the microfilament system can be viewed as an integral part of the physical barrier that separates the cellular contents from the outside world.
One of the first submembraneous actin-containing superstructures to be characterized was the red blood cell cortical network consisting of actin and the actin-binding proteins tropomyosin, spectrin and band 4.1 (29). Linkage to the plasma membrane is provided by the spectrin-binding protein ankyrin, which binds to the transmembrane anion channel band III, and band 4.1 (an ERM protein, see below), which interacts directly with the transmembrane protein glycophorin. The adaptability of the shape of the red blood cell to the compressive forces present in the low-bore blood capillaries reflects the elastic properties of the spectrin:actin network. In the intestinal epithelium (30, 31, and refs. therein), microvilli on the apical surface of the cell are formed by tightly packed actin filament bundles stabilized by the actin crosslinking proteins villin and fimbrin (Fig. 1). The presence of nonmuscle myosin in these structures suggests that active movement may be involved in the resorptive process.
Figure 1. Structural organization of a microvillus. Among the simplest and best studied cell surface structures are the microvilli on polarized epithelial cells, especially those on cells of the intestinal epithelium. The majority of the proteins involved in the architecture of the microvillus have now been identified and characterized. Most of them are essential for the survival of the organism. In some cases their three-dimensional structure has been determined. It is still unclear how the assembly of the microvillus takes place and how it is controlled, how dynamic microvilli are, and what their contribution to uptake of nutrients is. The construction of a microvillus on apical surface of an intestinal epithelial cell is shown here. Many of the microvillar proteins also occur in other cell surface structures. An exception is villin, which is confined to the microvilli of a few cell types. In addition to villin, the major actin-binding proteins of the microvillar core are ezrin, fimbrin, and myosin-I.
Specializations of the membrane-associated actomyosin system are found in all sensory organs, ie chemomechanical transduction in the actomyosin system is essential to the ability of the organism to perceive perturbations in the environment. For instance, the cellular basis for the sense of hearing lies in the microfilament-rich stereocilia in the hair cells of the inner ear, which convert subtle changes in the ambient air pressure to electrical signals transmitted to the brain via nerve impulses (32). Similarly, the mechanosensory cells in the skin confer the sense of touch (33).
Macrophages, lymphocytes, and neutrophils, which are highly motile cells, exploit the full potential of the cortical microfilament system as they forcibly move through and along extracellular matrices. When macrophages contact foreign invaders, the versatility of the cell cortex allows them to adapt their own surface structures as they engulf the pathogen.
The process of cell migration depends on the coordination of four major events involving microfilament reorganization: extension at the leading edge of membrane lamellae and filopodia, attachment to extracellular structures via transmembrane adhesion molecules, development of intracellular tension, and release of trailing end attachments with retraction of trailing ends (18, 27, 34, 35). The protrusion of lamellae at the advancing edge is driven by polymerization of actin filaments at focal complexes containing their fast growing ends at the plasma membrane, and crosslinking of formed filaments (24, 25, 36, 37). Clustering of transmembrane adhesion proteins, integrins, gives rise to focal adhesion sites (Fig. 2) linking extracellular matrix proteins to bundled actin filaments on the inside of the plasma membrane (38). The importance of the focal adhesions in transmembrane signalling is emphasized by their association with kinases regulating signal transducing protein:protein interactions and enzymes generating second messengers controlling not only the microfilament system, but also gene transcription (39). Several actin-binding proteins are targetted to these sites, contributing to formation of the integrin:actin filament linkage. The association of linkage proteins like talin, tensin, and vinculin appears to be controlled by phosphorylation. Myosin is activated through![]() -dependent processes (40, 41), and the regulated deployment of myosin molecules leads to the generation of the forces needed for cell motility and other translocation processes. Release of adhesion at the trailing end of a migrating cell is controlled by Ca and is followed by endocytosis of the integrins and their recycling to advancing cell edges (42). Used filaments are depolymerized, a process which involves the actin monomer- and filament-binding protein ADF (or cofilin) (17), and sequestered forms of unpolymerized actin are reformed (see Fig. 3).
-dependent processes (40, 41), and the regulated deployment of myosin molecules leads to the generation of the forces needed for cell motility and other translocation processes. Release of adhesion at the trailing end of a migrating cell is controlled by Ca and is followed by endocytosis of the integrins and their recycling to advancing cell edges (42). Used filaments are depolymerized, a process which involves the actin monomer- and filament-binding protein ADF (or cofilin) (17), and sequestered forms of unpolymerized actin are reformed (see Fig. 3).
Figure 2. Proteins of focal adhesion sites. Adherens junctions comprise both cell:extracellular matrix contacts, exemplified by focal adherens in tissue cultured cells, and intercellular junctions such as the zonula adherens between epithelial cells. The regulated assembly of adherens junctions is essential to a diversity of cellular functions including wound healing, anchorage-dependent cell proliferation, adhesion, and migration. Assembly of an adherens junction may be initiated when an integrin of the b1 subfamily binds to its extracellular matrix ligand, when a cadherin recognizes an identical molecule on a neighboring cell, or when a growth factor activates its receptor. These ligand-receptor interactions result in establishment of a physical connection between the extracellular ligand and the actin cytoskeleton. Efforts to elucidate these linkages have implicated vinculin as one of the connecting molecules in a chain that involves binding of the b1 subunit of integrin to talin, talin to vinculin, vinculin to a-actinin, and a-actinin to filamentous actin. It is now known that vinculin also can bind directly to actin filaments. How the initial ligand-receptor interactions trigger recruitment and organization of the multiple proteins found at an adherens junction is unknown, but recent evidence suggest that the activities of tyrosine kinases, tyrosine phosphatases, protein kinases and members of the Rho family of small GTPases are involved.
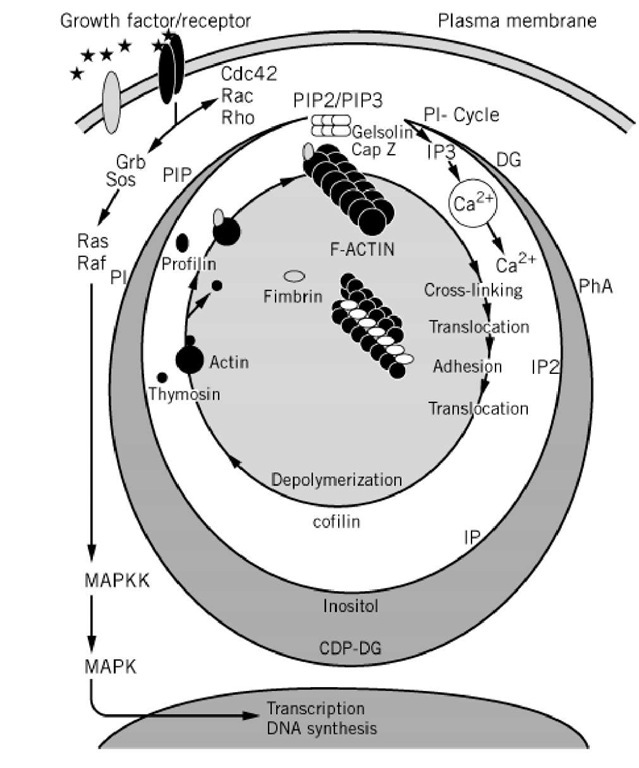
Figure 3. Links between the phosphatidylinositol and the cell motility cycles. Binding of growth factors like platelet-derived growth factor and epidermal growth factor to their cognate receptors activate the kinases activities of the receptors resulting in phosphorylation of a number of tyrosines on the cytoplasmic part of the receptor. This leads to activation of the signal transduction elements Cde42, Rac and Rho which control outgrowth of filopodia, membrane lamellae and the formation of stress fibers, respectively (64). There is only little information so far regarding the mechanism of activation of these small GTPases and how they relay the signal to the phosphatidylinositol (PI) cycle. Receptor activation is followed by an immediate increase in the activity of kinases that form PtdIns 4,5-bisphosphate (PIP2) and PtdIns 3,4,5-trisphosphate (PIP3). Profilin, gelsolin, CapZ, a-actinin, vinculin, the ERM family of proteins all bind these phosphoinositides, which influence their activity in the microfilament-based cell motility cycle (CM-cycle). Phosphoinositides binding to CapZ or gelsolin unblock the (+)-end of actin filaments allowing incorporation of profilin:actin at that site. Profilin:actin is then dissociated and the actin monomer becomes stably incorported into the growing filament. Cross-linking of filaments by proteins like fimbrin gives rise to the ensembles of filaments seen in membrane lamellae and filopodia which function together with myosin in the contractile phase of cell motility. Calcium ions play important roles in regulating both the actomyosin interaction and the depolymerization of actin filaments. The depolymerization is facilitated by the dephosphorylated form of cofilin and the actin monomer sequestering proteins thymosin and profilin (rev. 163). Another branch of signal transduction from the growth factor receptor activates the gene program that leads to DNA synthesis and cell division. Grb and Sos are small proteins acting as signal transduction elements activating the small GTPases Ras, which in turn activates the serine/threonine kinase Raf. The further signalling pathway is complex invlving mitogen activated protein kinases, MAPKK and MAPK (for review, see Cell 1998, 95, 447-450). Other abbreviations: IP3, inositol trisphosphate; IP2, inositol bisphosphate; IP, inositol monophosphate; DG, diacylglycerol; Pha, phosphatidic acid; CDP-DG, cytidyldiphosphate-diacylglycerol; PI, phosphatidylinositol.
Thus, the combined action of protein factors controlling polymerization, crosslinking and tension development in the cell cortex is initiated by transmembrane signalling. Controlling the timing and position of sites of polymerization at the cell surface is a potent means of regulating shape change. An astonishing variety of well-ordered, yet dynamic, structures are possible, reflecting the mechanical properties and specificities of the different kinds of actin bundling proteins and junctional connectors. Their timed and coordinated formation prevents the appearance of random rigid networks, which would be incompatible with the well-orchestrated movements seen.