The process of vacuum coating is used to modify a surface by evaporating a coating material under vacuum and condensing it on the surface. It is normally carried out under high vacuum conditions (at approximately 1 millionth of an atmosphere pressure). The material to be evaporated is heated until its vapor pressure appreciably exceeds the residual pressure within the vacuum system.
Vacuum coating can be used for many applications. For example, optical lenses are coated with magnesium fluoride to a fraction of a wavelength to prevent glare and provide much better transmission of light and a more reliable optical system. The deposited film is extremely adherent and will withstand normal cleaning.
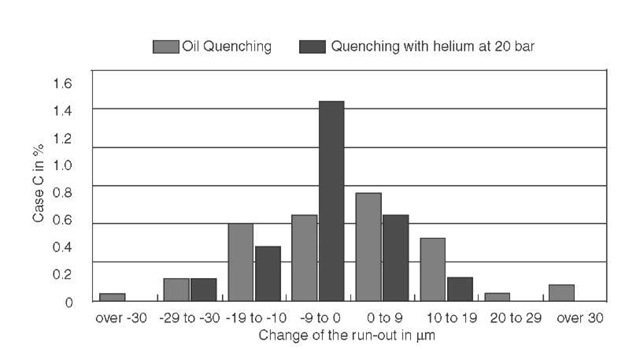
FIGURE V.1 Comparison of run-out between case hardening in the vacuum furnace with gas quenching and protective-gas carburizing with oil quenching.
Silicon monoxide is frequently used as an abrasion-resistant coating material. As deposited, it is soft and requires postheat treatment in air to convert it to silicon dioxide, which is transparent and extremely hard. It is frequently used to protect front surface mirrors and increases abrasion resistance by a factor of 5000 to 10,000, while maintaining equal or higher reflectivity. Similarly, titanium is sometimes used for coating and is subsequently oxidized to yield a titanium dioxide abrasion-resistant surface.
By far the most common type of vacuum coating is the process of vacuum metallizing. In this process metal is evaporated and used as deposited without further treatment as opposed to the evaporation of compounds or materials that require posttreatment.
Vacuum metallizing has generally been used as a decorative process whereby costume jewelry, toys, etc. are given a metallic sheen and are made highly reflective. The base material may be either plastic or metal. In either case, the part is frequently lacquered before metallizing to prevent the evolution of gas from the base and to provide a smooth surface without mechanical buffing. Because the metal deposit is only about 2 or 3 millionths of an inch thick, the smooth surface is necessary to give a specular reflection.
When the metal is on the outside of the coated part, it is referred to as front surface. However, in applications where it is used on the back of a transparent plastic (e.g., dashboards and taillight assemblies on automobiles) it is referred to as a second surface coating. The advantage of second surface coating is provided by using the plastic as the exposed surface. Front surface coatings must generally be protected with a transparent lacquer overcoat (applied after metallizing) because the thin decorative coatings are not wear resistant in themselves.
Aluminum is the most popular vacuum-metallizing coating material for most applications. However, other metals may be used, such as zinc, cadmium, copper, silver, gold, or chromium. Of all these metals, aluminum has the best general combination of reflectivity, conductivity, and stability in air. By adding color to the topcoat lacquer, the aluminum deposit may be made to appear like copper or gold as well as metallic sheens of blues, reds, yellow, etc. By using separate sources for each constituent, it is possible to deposit alloys as well as pure metals.
The above applications are for parts produced by batch metallizing; i.e., the individual parts are mounted on racks inserted in the vacuum system and after the necessary vacuum and evaporation temperatures are obtained, the parts are rotated so that they are uniformly coated by the evaporating metal. In batch metallizing the aluminum is evaporated from tungsten filaments, which are heated by direct resistance. Because of this, the amount of aluminum that can be charged is limited and only thin coatings can be produced. Similarly, only small surfaces (a few square centimeters), such as can be exposed within a matter of seconds, can be metallized.
When it is desirable to coat larger surfaces, e.g., rolls of flexible material, a semicontinuous metallizing process must be employed. For semicontinuous metallizing a roll of material is mounted in the vacuum chamber and unrolled under vacuum to coat either or both sides of the web, which is subsequently rewound in vacuum. This process is currently in use for coating rolls of plastic sheeting and paper. To coat continuously over a period of hours, it is necessary to have larger volumes of aluminum available for evaporation than can be held on resistance-heated tungsten filaments. Therefore, the aluminum is generally heated by induction in crucibles.
Coating of rolls of materials provided one of the first functional applications for coatings that used the electrical conductivity of the metal deposited. This conductive layer was deposited on thin insulating layers of either paper or plastic and could be used for winding miniature condensers. The electrical conductivity is also used in the metallizing process itself as a means of measuring the amount of metal deposited. Since the conductivity is a function of the thickness of the metal, continuously measuring conductivity provides a control for the amount of metal deposited. Other functional uses of the coating are based on its reflectivity (e.g., reflective insulation).
Vacuum metallizing has recently been extended to include thick films, i.e., in the range of 1 to 3 mils. Such coatings serve as corrosion-resistant barriers, particularly on high-tensile-strength steel exposed to marine atmospheres. Where the temperature requirements of steel are less than 260°C, cadmium deposits can be used. For temperatures in excess of this, aluminum shows much better protection and does not react with the base steel as cadmium does.
Truly continuous operation is necessary for coating rolled steel. Here the rolls are unwound and rewound in air with the strip passing through seals into the vacuum chamber where it is coated. The metallizing of rolled stock allows separate control on each side of the web and the composition of the coating, as well as thickness, may be changed from one side to the other. There are several typical advantages of vacuum metallizing:
1. Close control of coating thickness and composition
2. Uniform deposits without buildup at sharp discontinuities
3. High coating rate
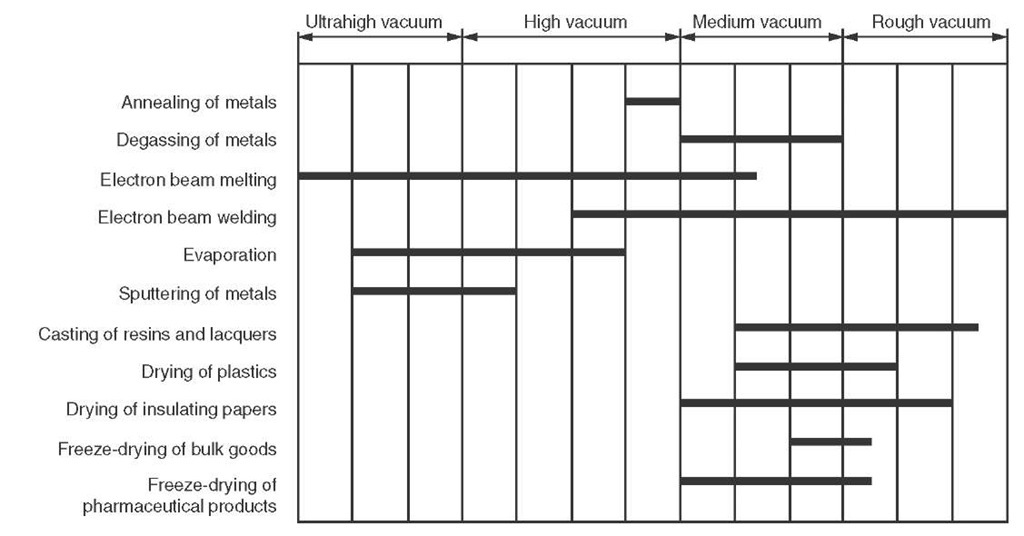
FIGURE V.2 Pressure ranges for various industrial processes.
4. Low coating costs in volume production
5. Long life of equipment since few moving parts
There are also disadvantages of the process:
1. Part must be extremely clean.
2. Surfaces to be metallized must not evolve gas under vacuum.
3. Parts must not be temperature sensitive, i.e., must be stable to about 125°C.
4. Deposits form well only on a surface exposed to hot metal; reentrant angles are not well coated.
The cost for metallizing in production lots for corrosion-resistant coatings is comparable to electroplating. Decorative metallizing is generally much less expensive than electroplating.