Energy Generation and Flow
This is ultimately limited by some basic physics, some of which we understand (Thermodynamics) and some of which we don’t (Chaos).
Even though a system may appear to be very simple, the behavior of that system might be chaotic. The elements of this theory are hard to describe but some neutrally buoyant helium balloons floating around class today can serve as an example.
In Class Chaos Demo
Helium balloons and a demonstration of the principles of chaos:
• Unstable equilibrium (a perturbation in either direction causes an irrecoverable situation)
• No predicative power, our neutrally buoyant helium ballon can go in any direction
• Random interactions will occur that would not otherwise occur (e.g. whose head is this balloon going to fall on).
• These random interactions will increase the chaos of the system (student x bats balloon in some direction).
• An external event which is not in the interactions reduces the chaos (e.g. the helium runs out and the balloon falls on the floor).
• The nature of the chaotic system is continuously changing –> i.e. its difficult to maintain the neutral buoyancy of the balloon. When its not neutrally buoyant, its less chaotic and more deterministic.
• In principle, the motions of the ballon are entirely governed by physics, hence if we knew all the physics we would have predictive power. However, the amount of information which is required to be known is nearly infinite.
So even though the helium balloon is a simple system, its motion and its interaction with other elements is chaotic.
Chaos seems to exist in a variety of natural systems to some extent or another
• The Flow of the McKenzie River
• The Greenhouse effect
• Hurricane evolution
• Planetary orbits
• Evolution of species
THERMODYNAMIC LAWS
You can not subvert or change these laws:
The Zeroth Law (0): Systems are in equilibrium when they are at the same temperature.
• System is in it lowest energy state
• No more energy can be extracted from it
• All systems of different temperature will tend to equilibrium when they are no longer thermally isolated.
Modelling Thermodynamic Equilibrium: (
Warning, JAVA applets Page (might kill some browsers); Suggest using Netscape 4.04 or IE 4.0 for this. Netscape 3.0 might not completey work.
The First Law (1): Energy is Conserved in a closed system:
• The net flow of energy across some system is equal to the change in energy of that system
• We usually consider work and heat flow as the two kinds of energy
The Second Law (2): The Law of Entropy:
• It is not possible to extract heat-energy from a reservoir and perform work without creating waste heat that does no work.
• The amount of disorder increases
• Things tend toward a state of randomness(this is not the same as chaos)
• You can not go from a disordered system to an ordered system without inputting more energy (this is the most important attribute of the second law)
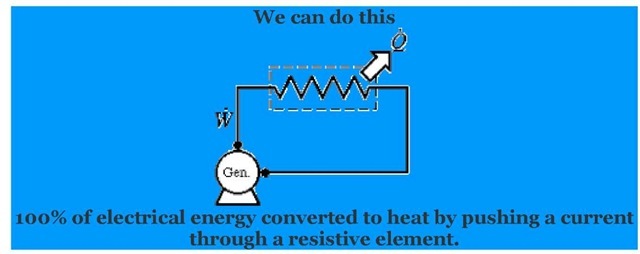
Example:
We can not reverse that process to do this:
In the course of doing the original work we have increased the disorder to the system by heating it. In no way can we recover work of that this disordered system without putting energy into the system.
To decrease local entropy requires work (energy).
• Your dorm room - it takes a lot of work to decrease the amount of disorder
• Iron Ore is originally concentrated in mountains – has a low disorder. Eventually it becomes mined and ends up distributed in the nations landfills.
• This combination of letters is readable and hence of low order but ticmiainflteriraaladecolwrerdoohsobntoftrsdbne is not and would require a lot of work to rearrange into the previous sentence
• Rich fossil fuel deposits are stored in a state of low order. when we liberate all of that so that the individual atoms become randomly distributed, where will society find the energy to maintain local order?
Energy From the Oceans
More promising technology is OTEC (Ocean Thermal Energy Generation). This takes advantage of the fact that the ocean is an enormous heat engine.
Physics of Heat Engines:
• efficiency = work done/energy input
• it can be shown that this is equivalent to efficiency (in %) = 1 – T1/T2 ; T1 < T2
T is measured in Kelvins
• So, in principle any two reservoirs with different temperatures T1 and T2 can produce energy. There will be a demonstration of this principle in class today.
Boiling water It^rT = 373K o Ice Water D3f>T=273K o Liquid Nitrogen T=77K
• Efficiency of Boiling water and Ice water: 1 – (273/373) = 27%
• Efficienty of Ice water and LN2: 1 – (77/273) = 72%
• What is efficiency of Boiling water and LN2?
Thermodynamic Constraints:
• Systems are in equilibrium when they are at the same temperature
• energy is conserved within a closed system
• it is not possible to extract heat energy from a reservoir and perform work without transferring heat to a reservoir of lower temperature. In other words, all thermodynamic systems must tend towards equilibrium. Some energy goes towards performing work and some is lost as waste heat.
To get the highest efficiency one wants to maximize the difference between T1 and T2 but then their are material problems (containers melt, freeze, etc)
Typical Case:
• Coal-fired burner: T = 825K
• Cooling tower: T = 300 K
• efficiency = 1 – 300/825 = 64%
How this all works:
• Exhaust steam is condensed back into liquid thereby decreasing its total volume by a factor of 1000
• Therefore the work done by the pump is down by a factor of 1000 compared to if it had to pump steam directly back into the system
Heat Energy from the Ocean Do this:
Basic principle is that heat difference is used to condense a steam into a liquid then return it to be reheated.
Since heat differences in the ocean will be smaller, then one must substitute ammonia for water as the working fluid.
Example Calculations:
• Surface Ocean temperature is 25 degrees C (298K)
• At 1000 meters depth the temperature is 5 degrees C (278K)
• Efficiency = 1 – 278/298 = .067 (6.7%)
• Power in cooling 1000 gallons of water per second by 2 degrees C is 32 MegaWatts (because water has such high heat capacity/storage)
• using 6.7% efficiency would then yield 2 Megawatts (1/500 typical coal-fired plant)
• But this is only for 1000 measly little gallons per second
OTEC Potential Sites:
• Florida, Puerto Rico, and Hawaii
• Indian Ocean
• Northeast Australia, Indonesia and Mexico
Above sites typically have thermal gradients higher then 22 degrees C
Energy extracted comes from the cooling of the warmer water this is transferred to the ammonia which does the actual work of turning the turbine (as ammonia steam)