Abstract
Exergy analysis is an assessment technique for systems and processes that is based on the second law of thermodynamics. Exergy analysis has been increasingly applied over the last several decades largely because of its advantages over energy analysis: more meaningful efficiencies are evaluated because exergy efficiencies are always a measure of the approach to the ideal and inefficiencies in a process are better pinpointed because exergy analysis quantifies the types, causes, and locations of losses. In this article, the role of exergy analysis in assessing and improving energy systems is examined. Also, exergy and its use as an analysis technique are briefly described, the range of energy systems that have been assessed with exergy analysis are surveyed, and several example applications of exergy analysis are presented, ranging from simple to complex.
INTRODUCTION
Energy analysis is based on the first law of thermodynamics, which embodies the principle of conservation of energy and is the traditional method used to assess the performance and efficiency of energy systems and processes.
Exergy analysis is a thermodynamic analysis technique for systems and processes that is based on the second law of thermodynamics. Exergy analysis has been increasingly applied over the last several decades, in large part because of its advantages over energy analysis:
• More meaningful efficiencies are evaluated with exergy analysis because exergy efficiencies are always a measure of the approach to the ideal.
• Inefficiencies in a process are better pinpointed with exergy analysis because the types, causes, and locations of the losses are identified and quantified.
In this article, the role of exergy analysis in the assessment and improvement of energy systems is examined. First, exergy and its use as an analysis technique are briefly described. Second, the ranges of energy systems that have been assessed with exergy analysis are surveyed. Third, several example applications of exergy analysis are presented, ranging from simple devices to large and complex systems.
EXERGY
Exergy can be regarded as a measure of the usefulness or quality of energy. Technically, exergy is defined as the maximum amount of work that can be produced by a stream of energy or matter, or from a system, as it is brought into equilibrium with a reference environment. Unlike energy, exergy is consumed during real processes due to irreversibilities and conserved during ideal processes. Exergy and related concepts have been recognized for more than a century.[1]
Exergy analysis is a methodology that uses the first and second laws of thermodynamics for the analysis, design, and improvement of energy and other systems.[2-14] The exergy method is useful for improving the efficiency of energy-resource use, for it quantifies the locations, types, and magnitudes of wastes and losses. In general, more meaningful efficiencies are evaluated with exergy analysis rather than energy analysis because exergy efficiencies are always a measure of the approach to the ideal. Therefore, exergy analysis accurately identifies the margin available to design more efficient energy systems by reducing inefficiencies.
In evaluating exergy, the characteristics of the reference environment must be specified,[2-15] usually by specifying the temperature, pressure, and chemical composition of the reference environment. The results of exergy analyses, consequently, are relative to the specified reference environment, which in most applications is modeled after the actual local environment. The exergy of a system is zero when it is in equilibrium with the reference environment. The tie between exergy and the environment has implications regarding environmental impact.[7'8'16]
The theory and the applications of exergy have been described in specialized topics, e.g.,[2-8] general thermodynamics texts, e.g.,[9'10] and journal articles e.g.[11-14] Many applications of exergy analysis have been reported in fields ranging from power generation1-17’18-1 and cogeneration[9] to district energy,[19] thermal processes,[20,21] and thermal energy storage[22,23] and on to systems as large as countries.[24,25]
Exergy and the Reference Environment
Exergy quantities are evaluated with respect to a reference environment. The intensive properties of the reference environment in part determine the exergy of a stream or system. The reference environment is in stable equilibrium, with all parts at rest relative to one another and with no chemical reactions occurring between the environmental components. The reference environment acts as an infinite system and is a sink and source for heat and materials. It experiences only internally reversible processes in which its intensive state remains unaltered (i.e., its temperature 7o, pressure Po, and the chemical potentials n,00 for each of the i components present remain constant). The exergy of the reference environment is zero. More information on reference-environment models can be found in this topic in an article by the present author entitled “Exergy: Environmental Impact Assessment Applications.”
Exergy Balances
Energy and exergy balances can be written for a general process or system.
Since energy is conserved, an energy balance for a system may be written as
Energy input — Energy output
= Energy accumulation (l)
Energy input and output refer respectively to energy entering and exiting through system boundaries. Energy accumulation refers to build-up (either positive or negative) of the quantity within the system.
By contrast, an exergy balance can written as
Exergy input — Exergy output — Exergy consumption
= Exergy accumulation (2)
This expression can be obtained by combining the principles of energy conservation and entropy nonconser-vation, the latter of which states that entropy is created during a process due to irreversibilities. Exergy is consumed due to irreversibilities, with exergy consumption proportional to entropy creation.
Eqs. 1 and 2 demonstrate an important difference between energy and exergy—energy is conserved while exergy, a measure of energy quality or work potential, can be consumed.
Definitions
It is helpful to define some terms related to exergy for readers. The following are exergy quantities:
Exergy: A general term for the maximum work potential of a system, a stream of matter, or a heat interaction in relation to the reference environment (see definition below) as the datum state; or the maximum amount of shaft work obtainable when a steady stream of matter is brought from its initial state to the dead state (see definition below) by means of processes involving interactions only with the reference environment.
Physical exergy: The maximum amount of shaft work obtainable from a substance when it is brought from its initial state to the environmental state (see definition below) by means of physical processes involving interaction only with the environment.
Chemical exergy: The maximum work obtainable from a substance when it is brought from the environmental state to the dead state by means of processes involving interaction only with the environment.
Thermal exergy: The maximum amount of shaft work obtainable from a given heat interaction using the environment as a thermal energy reservoir.
Exergy consumption: The exergy consumed during a process due to irreversibilities within the system boundaries.
The following terms relate to the reference environment and its state:
Reference environment: An idealization of the natural environment, which is characterized by a perfect state of equilibrium, i.e., absence of any gradients or differences involving pressure, temperature, chemical potential, kinetic energy, and potential energy. The reference environment constitutes a natural reference medium with respect to which the exergy of different systems is evaluated.
Dead state: The state of a system when it is in thermal, mechanical, and chemical equilibrium with a conceptual reference environment, which is characterized by a fixed pressure, temperature, and chemical potential for each of the reference substances in their respective dead states.
Environmental state: The state of a system when it is in thermal and mechanical equilibrium with the reference environment, i.e., at the pressure and temperature of the reference environment.
Reference state: A state with respect to which values of exergy are evaluated. Several reference states are used, including environmental state, dead state, standard environmental state, and standard dead state.
EXERGY ANALYSIS
Exergy analysis involves the application of exergy concepts, balances, and efficiencies to evaluate and improve energy and other systems. Many engineers and scientists suggest that devices are best evaluated and improved upon using exergy analysis in addition to or in place of energy analysis.
A journal devoted to exergy matters entitled The International Journal of Exergy has recently been established by Inderscience.
A simple procedure for performing energy and exergy analyses involves the following steps:
• Subdivide the process under consideration into as many sections as desired, depending on the depth of detail and the understanding desired from the analysis.
• Perform conventional mass and energy balances on the process and determine all basic quantities (e.g., work, heat) and properties (e.g., temperature, pressure).
• Based on the nature of the process, the acceptable degree of analysis complexity and accuracy, and the questions for which answers are sought, select a reference-environment model.
• Evaluate energy and exergy values relative to the selected reference-environment model.
• Perform exergy balances, including the determination of exergy consumptions.
• Select efficiency definitions depending on the measures of merit desired and evaluate the efficiencies.
• Interpret the results and draw appropriate conclusions and recommendations relating to such issues as design changes, retrofit plant modifications, etc.
EXERGY ANALYSIS AND EFFICIENCY
Increases in efficiency are subject to two constraints, which are often poorly understood:
• Theoretical limitations, which establish the maximum efficiency theoretically attainable for a process by virtue of the laws of thermodynamics
• Practical limitations, which further limit increases in efficiency
First, consider practical limitations on efficiency. In practice, the goal when selecting energy sources and utilization processes is not to achieve maximum efficiency, but rather to achieve an optimal trade-off between efficiency and such factors as economics, sustainability, environmental impact, safety, and societal and political acceptability. This optimum is dependent on many factors controllable by society. Furthermore, these factors can be altered to favor increased efficiency (e.g., governments can offer financial incentives that render high-efficiency technologies economically attractive or provide disincentives for low-efficiency alternatives through special taxes and regulations).
Next, consider theoretical limitations on efficiency, which must be clearly understood to assess the potential for increased efficiency. Lack of clarity on this issue in the past has often led to confusion, in part because energy efficiencies generally are not measures of how nearly the performance of a process or device approaches the theoretical ideal. The consequences of such confusion can be significant. For example, extensive resources have at times been directed towards increasing the energy efficiencies of devices that in reality were efficient and had little potential for improvement. Conversely, devices at other times have not been targeted for improved efficiency even though the difference between the actual and maximum theoretical efficiencies, which represents the potential for improvement, has been large.
The difficulties inherent in energy analysis are also attributed to the fact that it only considers quantities of energy and ignores energy quality, which is continually degraded during real processes. Exergy analysis overcomes many of the problems associated with energy analysis.
OVERVIEW OF EXERGY ANALYSIS APPLICATIONS
Exergy analysis has been applied to a wide range of processes and systems, including those that are mechanical, thermal, electrical, and chemical. The types of applications of exergy methods that have been reported over the last several decades include:
• Electricity generation using both conventional devices such as fossil and nuclear power plants as well as alternative devices such as fuel cells and solar energy systems.
• Energy storage systems such as batteries, pumped storages, and thermal energy storages.
• Combustion technologies and systems and engines of various types.
• Transportation systems for land, air, and water transport.
• Heating and cooling systems for building systems and industrial applications.
• Cogeneration systems for producing heating and electrical needs simultaneously.
• Chemical processes such as sulphuric acid production, distillation, and water desalination, as well as petrochemical processing and synthetic fuels production.
• Metallurgical processes such as lead smelting.
EXAMPLES OF EXERGY ANALYSIS APPLICATIONS
Three examples of differing complexity of applications of exergy analysis are presented:
• An electrical resistance space heater (a simple component)
• A thermal energy storage system (a simple system containing a number of components)
• A coal-fired electrical generating station (a complex system)
Electrical Resistance Space Heater
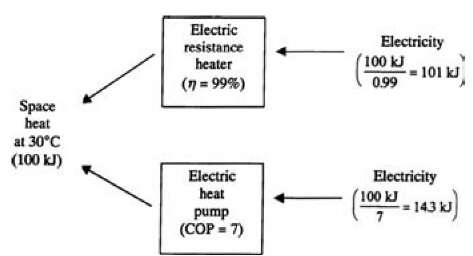
An electrical resistance space heater converts electricity to heat at a temperature suitable for room comfort, and is illustrated in Fig. 1 (Part A).
The energy efficiency of electric resistance space heating often exceeds 99%, implying that the maximum possible energy efficiency for electric resistance heating is 100%, corresponding to the most efficient device possible.
This understanding is erroneous; however, energy analysis ignores the fact that in this process, high-quality energy (electricity) is used to produce a relatively low-quality product (warm air). Exergy analysis recognizes this difference in energy qualities and indicates the exergy of the heat delivered to the room to be about 5% of the exergy entering the heater. Thus, the exergy efficiency of electric resistance space heating is about 5%.
The exergy results are useful. Since thermodynamically ideal space heating has an exergy efficiency of 100%, the same space heating can in theory be achieved using as little as 5% of the electricity used in conventional electric resistance space heating. In practical terms, one can achieve space heating with a greatly reduced electricity input using an electric heat pump (see Part B of Fig. 1), using 15% of the electricity that electric resistance heating would require, for a heat pump with a “coefficient of performance” of 7.
Fig. 1 Comparison of the quantity of electricity required to provide 100 kJ of space heat using two different heating devices: (a) an electric resistance heater and (b) an electric heat pump. Here, r| denotes energy efficiency and COP coefficient of performance.
Thermal Storage System
A thermal energy storage system receives thermal energy and holds it until it is required. Thermal storages can store energy at temperatures above or below the environment temperature, and they come in many types (e.g., tanks, aquifers, ponds, caverns).
The evaluation of a thermal energy storage system requires a measure of performance which is rational, meaningful, and practical. The conventional energy storage efficiency is inadequate. A more perceptive basis is needed if the true usefulness of thermal storages is to be assessed and their economic benefit optimized, and exergy efficiencies provide such performance measures.
The notion that energy efficiency is an inappropriate measure of thermal storage performance can be illustrated. Consider a perfectly insulated thermal storage containing 1000 kg of water, initially at 40°C. The ambient temperature is 20°C and the specific heat of water is taken to be constant at 4.2 kJ/kg K. A quantity of 4200 kJ of heat is transferred to the storage through a heat exchanger from an external body of 100 kg of water cooling from 100 to 90°C. This heat addition raises the storage temperature 1.0°C to a value of 41°C. After a period of storage, 4200 kJ of heat are recovered from the storage through a heat exchanger, which delivers it to an external body of 100 kg of water, raising the temperature of that water from 20 to 30°C. The storage is returned to its initial state at 40°C.
For this storage cycle, the energy efficiency—the ratio of heat recovered from the storage to heat injected—is 4200/4200 kJ = 1, or 100%. But the recovered heat is at only 30°C and is of little use, having been degraded even though the storage energy efficiency was 100%. The exergy recovered in this example is 70 kJ and the exergy supplied 856 kJ. Thus the exergy efficiency, the ratio of the thermal exergy recovered from storage to that injected, is 70/856 = 0.082 or 8.2%, a much more meaningful expression of the achieved performance.
Consequently, a device which appears to be ideal on an energy basis is correctly shown to be far from ideal on an exergy basis, clearly demonstrating the benefits of using exergy analysis for evaluating thermal storage.
Coal-Fired Electrical Generating Station
Energy and exergy analyses are applied to the Nanticoke coal-fired electrical generating station in Ontario, Canada, which has a net unit electrical output of approximately 500 MWe and is operated by the provincial electrical utility, Ontario Power Generation (formerly Ontario Hydro). This example illustrates how exergy analysis allows process inefficiencies to be better pinpointed than an energy analysis does and how efficiencies are to be more rationally evaluated.
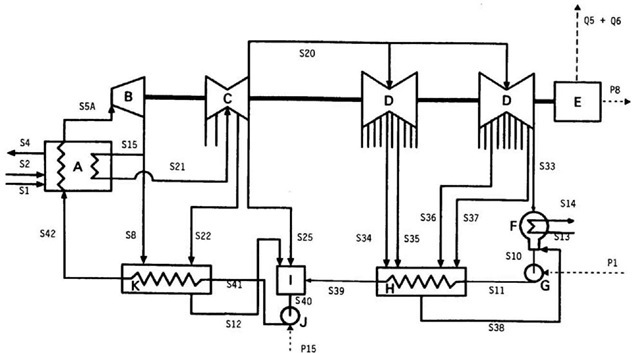
A detailed flow diagram for a single unit of the station is shown in Fig. 2. The symbols identifying the streams are described in Table 1a-c for material, thermal, and electrical flows, respectively, with corresponding data. Fig. 2 has four main sections:
• Steam Generation. Eight pulverized-coal-fired natural circulation steam generators each produce 453.6 kg/s steam at 16.89 MPa and 538°C and 411.3 kg/s of reheat steam at 4.00 MPa and 538°C. Air is supplied to the furnace by two 1080 kW 600-rpm motor-driven forced draft fans. Regenerative air preheaters are used. The flue gas passes through an electrostatic precipitator rated at 99.5% collection efficiency and exits the plant through two multiflued, 198 m high chimneys.
• Power Production: The steam passes through a series of turbine generators linked to a transformer. Extraction steam from several points on the turbines preheats feedwater in several low- and high-pressure heat exchangers and one spray-type open deaerating heat exchanger. The low-pressure turbines exhaust to the condenser at 5 kPa. Each station unit has a 3600-rpm, tandem-compound, impulse-reaction turbine generator containing one single-flow high-pressure cylinder, one double-flow intermediate-pressure cylinder, and two double-flow low-pressure cylinders. Steam exhausted from the high-pressure cylinder is reheated in the combustor.
• Condensation: Cooling water from Lake Erie condenses the steam exhausted from the turbines. The cooling-water flow rate is adjusted to achieve a specified cooling-water temperature rise across the condenser.
• Preheating: The temperature and pressure of the feedwater are increased in a series of pumps and feedwater-heater heat exchangers.
The reference-environment model used here has a temperature of 15°C (the approximate mean temperature of the lake cooling water), a pressure of 1 atm, and a chemical composition consisting of air saturated with water vapor, and the following condensed phases at 15° C and 1 atm: water (H2O), gypsum (CaSC>4 • 2H2O), and limestone (CaCO3). For simplicity, heat losses from external surfaces are assumed to occur at the reference-environment temperature of 15°C.
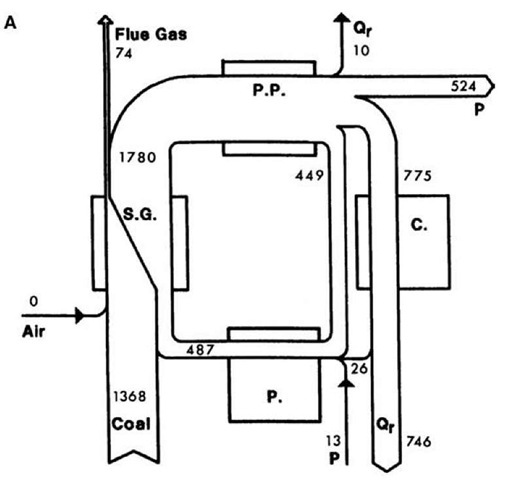
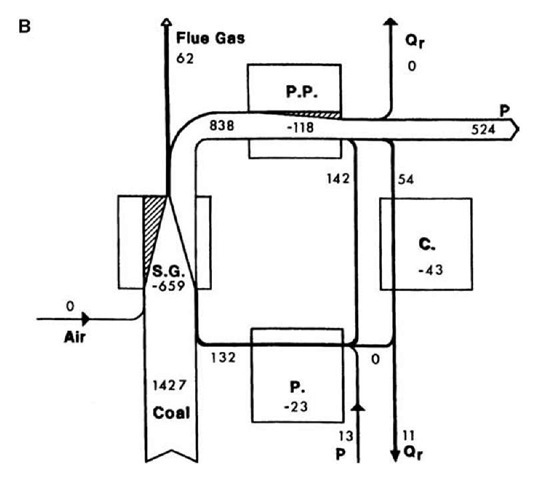
Energy and exergy values for the streams identified in Fig. 2 are summarized in Table 1. Exergy-consumption values for the devices are listed, according to process section, in Table 2. Fig. 3 a and b illustrate the net energy and exergy flows and exergy consumptions for the four main process sections.
Fig. 2 A unit of the coal-fired electrical generating station. Lines exiting the turbines represent extraction steam. The station has four main sections: Steam Generation (Device A), Power Production (B-E), Condensation (F), and Preheating (G-K). The external inputs for Device A are coal and air, and the output is stack gas and solid waste. The external outputs for Device E are electricity and waste heat. Electricity is input to Device G and Device J and cooling water enters and exits Device F. A: steam generator and reheater, B: high-pressure turbine, C: intermediate-pressure turbine, D: low-pressure turbines, E: generator and transformer, F: condenser, G: hot well pump, H: low-pressure heat exchangers, I: open deaerating heat exchanger, J: boiler feed pump, K: high-pressure heat exchangers.
Table 1a Data for material flows for a unit of the coal-fired electrical generating station
| Mass flow Temperature Pressure | Energy flow | Exergy flow | ||||
| Stream | rate (kg/s)a | (° C) | (N/m2) | Vapor frac. | rate (MW) | rate (MW) |
| S1 | 41.74 | 15.00 | 1.01 X 10s | solid | 1367.58 | 1426.73 |
| S2 | 668.41 | 15.00 | 1.01 X 10s | 1.0 | 0.00 | 0.00 |
| S3C | 710.15 | 1673.59 | 1.01 X 10s | 1.0 | 1368.00 | 982.85 |
| S4 | 710.15 | 119.44 | 1.01 X 10s | 1.0 | 74.39 | 62.27 |
| S5A | 453.59 | 538.00 | 1.62 X107 | 1.0 | 1585.28 | 718.74 |
| S8 | 42.84 | 323.36 | 3.65 X106 | 1.0 | 135.44 | 51.81 |
| S10 | 367.85 | 35.63 | 4.50 X103 | 0.0 | 36.52 | 1.20 |
| S11 | 367.85 | 35.73 | 1.00 X106 | 0.0 | 37.09 | 1.70 |
| S12 | 58.82 | 188.33 | 1.21 X106 | 0.0 | 50.28 | 11.11 |
| S13 | 18,636.00 | 15.00 | 1.01 X 10s | 0.0 | 0.00 | 0.00 |
| S14 | 18,636.00 | 23.30 | 1.01 X 10s | 0.0 | 745.95 | 10.54 |
| S15 | 410.75 | 323.36 | 3.65 X106 | 1.0 | 1298.59 | 496.81 |
| S20 | 367.85 | 360.50 | 1.03 X106 | 1.0 | 1211.05 | 411.16 |
| S21 | 410.75 | 538.00 | 4.00 X106 | 1.0 | 1494.16 | 616.42 |
| S22 | 15.98 | 423.23 | 1.72 X106 | 1.0 | 54.54 | 20.02 |
| S25 | 26.92 | 360.50 | 1.03 X106 | 1.0 | 88.64 | 30.09 |
| S33 | 309.62 | 35.63 | 4.50 X103 | 0.93 | 774.70 | 54.07 |
| S34 | 10.47 | 253.22 | 3.79 X 10s | 1.0 | 32.31 | 9.24 |
| S35 | 23.88 | 209.93 | 2.41 X 10s | 1.0 | 71.73 | 18.82 |
| S36 | 12.72 | 108.32 | 6.89 X104 | 1.0 | 35.77 | 7.12 |
| S37 | 11.16 | 60.47 | 3.45 X104 | 1.0 | 30.40 | 5.03 |
| S38 | 58.23 | 55.56 | 1.33 X104 | 0.0 | 11.37 | 0.73 |
| S39 | 367.85 | 124.86 | 1.00 X106 | 0.0 | 195.94 | 30.41 |
| S40 | 453.59 | 165.86 | 1.00 X106 | 0.0 | 334.86 | 66.52 |
| S41 | 453.59 | 169.28 | 1.62 X107 | 0.0 | 347.05 | 77.57 |
| S42 | 453.59 | 228.24 | 1.62 X107 | 0.0 | 486.75 | 131.93 |
aThe composition of all streams is 100% H2O, except that, on a volume basis, the composition of S1 is 100% carbon, of S2 is 79% N2 and 21% O^ and of both S3 and S4 is 79% N2, 6% O2, and 15% CO2.
bVapor fraction is listed as 0.0 for liquids and 1.0 for superheated vapors.
Overall energy and exergy efficiencies are evaluated as
Energy efficiency = (Net energyoutputwith electricity)/(Energy input) (3)
and
Exergy efficiency = (Net exergy outputwith electricity)/(Exergy input) (4)
Coal is the only input source of energy or exergy and the energy and exergy efficiencies are 37 and 36%, respectively. The small difference in the efficiencies is due to the fact that the specific chemical exergy of coal is slightly greater than its energy. Although the station energy and exergy efficiencies are similar, these efficiencies differ markedly for many station sections.
Table 1b Data for principal thermal flows for a unit of the coal- Table 1c Data for principal electrical flows for a unit of the fired electrical generating station coal-fired electrical generating station
| Energy flow rate | Exergy flow rate | Stream | Energy (and exergy) flow rate (MW) | |
| Stream | (MW) | (MW) | P1 | 0.57 |
| Q5 | 5.34 | 0.00 | P8 | 523.68 |
| Q6 | 5.29 | 0.00 | P15 | 12.19 |
Table 2 Breakdown of exergy consumption rates for a unit of the coal-fired electrical generating station
| Section/device | Exergy consumption rate (MW) |
| Steam generation | |
| Steam generator (including | 659.0 |
| combustor) | |
| 659.0 | |
| Power production | |
| High-pressure turbine | 26.4 |
| Intermediate-pressure | 22.3 |
| turbine | |
| Low-pressure turbines | 59.2 |
| Generator | 5.3 |
| Transformer | 5.3 |
| 118.5 | |
| Condensation | |
| Condenser | 43.1 |
| 43.1 | |
| Preheat | |
| Low-pressure heat | 10.7 |
| exchangers | |
| Deaerating heat exchanger | 5.1 |
| High-pressure heat | 6.4 |
| exchangers | |
| Hot well pumps | 0.1 |
| Boiler feed pumps | 1.1 |
| 23.4 | |
| Total | 844.0 |
In the Steam Generation section, exergy consumptions are substantial, accounting for 659 MW (or 72%) of the 916 MW station exergy loss. Of this 659, 444 MW is consumed with combustion and 215 MW with heat transfer. The energy and exergy efficiencies for the Steam Generation section, considering the increase in energy or exergy of the water as the product, are 95 and 49%, respectively. The Steam Generation section thus appears significantly more efficient on an energy basis than on an exergy basis. Physically, this discrepancy implies that although 95% of the input energy is transferred to the preheated water, the energy is degraded as it is transferred. Exergy analysis highlights this degradation.
In the condensers, a large quantity of energy enters (775 MW for each unit), of which close to 100% is rejected, while a small quantity of exergy enters (54 MW for each unit), of which about 25% is rejected and 75% is internally consumed. Thus, energy analysis leads to the erroneous conclusion that almost all losses in electricity-generation potential for the station are associated with the heat rejected by the condensers, while exergy analysis demonstrates quantitatively and directly that the condensers are responsible for little of these losses (see Fig. 3b). This discrepancy arises because heat is rejected by the condensers at a temperature very near that of the environment.
In the Power Production and Preheating sections, energy losses are small (less than 10 MW) and exergy losses moderately small (118 MW in Power Production and 23 MW in Preheating). The exergy losses are almost completely associated with internal consumptions.
In assessing the thermodynamic characteristics of a coal-fired electrical generating station, several illuminating insights into performance are acquired:
• Although energy and exergy efficiencies are similar for the station, energy analysis does not identify the location and cause of process inefficiencies, while exergy analysis does. Energy losses are associated with emissions (mainly heat rejected by condensers) and exergy losses are primarily associated with consumptions (mainly in the combustors).
• Because devices with the largest thermodynamic losses have the largest margins for efficiency improvement, efforts to increase the efficiencies of coal-fired electrical generating stations should focus on the combustors. For instance, technologies capable of producing electricity without combustion (e.g., fuel cells) or utilizing heat at high temperatures could increase efficiencies significantly. This suggestion is, of course, overly simplistic, as such decisions must also account for other technical and economic factors.
• The use of heat rejected by condensers only increases the exergy efficiencies by a few percent.
APPLICATIONS BEYOND THERMODYNAMICS
Exergy concepts can be applied beyond thermodynamics in such fields as environmental impact assessment,[7,8,16]
[5,17,20,26] . ,• [27] economics, and policy.
Exergy and Environment
Many suggest that the impact of energy utilization on the environment is best addressed by considering exergy. Although the exergy of an energy form or a substance is a measure of its usefulness, exergy is also a measure of its potential to cause change. The latter point suggests that exergy may be or may provide the basis for an effective measure of the potential of a substance or energy form to impact the environment. The relation between exergy and the environment is discussed in this topic in an article entitled “Exergy: Environmental Impact Assessment Applications.”
Fig. 3 (A) Diagram for a coal-fired electrical generating station unit indicating net energy flow rates (MW) for streams. Stream widths are proportional to energy flow rates. Station sections shown are Steam Generation (S.G.), Power Production (P.P.), Condensation (C.) and Preheating (P.). Streams shown are electrical power (P), heat input (Q), and heat rejected (Qr). (B) Diagram for a coal-fired electrical generating station unit indicating net exergy flow rates for streams and consumption rates (negative values) for devices (in MW). Stream widths are proportional to exergy flow rates and shaded regions to exergy consumption rates. Other details are as in (A).
Exergy and Economics
Another area in which applications of exergy are increasing is that of economics. In the analysis and design of energy systems, techniques are often used that combine scientific disciplines like thermodynamics with economics to achieve optimum designs. For energy systems, costs are conventionally based on energy. Many researchers, however, have recommended that costs are better distributed among outputs based on exergy. Methods of performing exergy-based economic analyses have evolved (e.g., thermoeconomics, second-law costing, and exergoe-conomics). These analysis techniques recognize that exergy, not energy, is the commodity of value in a system, and assign costs and prices to exergy-related variables. These techniques usually help in appropriately allocating economic resources so as to optimize the design and operation of a system and its economic feasibility and profitability (by obtaining actual costs of products and their appropriate prices).
CONCLUSION
Exergy analysis provides information that influences design, improvement, and application decisions and it is likely to be increasingly applied. Exergy also provides insights into the “best” directions for research, where “best” is loosely considered most promising for significant efficiency gains. There are two main reasons for this conclusion:
• Unlike energy losses, exergy losses represent true losses of the potential to generate the desired product from the given driving input. Focusing on exergy losses permits research to aim at reducing the losses that degrade efficiency.
• Unlike energy efficiencies, exergy efficiencies always provide a measure of how closely the operation of a system approaches the ideal. By focusing research on plant sections or processes with the lowest exergy efficiencies, effort is directed to those areas that inherently have the largest margins for efficiency improvement. By focusing on energy efficiencies, on the other hand, research can inadvertently be expended on areas for which little margins for improvement exist, even theoretically.
Exergy analysis results typically suggest that improvement efforts should concentrate more on internal rather than external exergy losses based on thermodynamic considerations, with a higher priority for the processes that have larger exergy losses. Of course, effort should still be devoted to processes having low exergy losses when cost-effective ways to increase efficiency can be identified.
Energy-related decisions should not be based exclusively on the results of energy and exergy analyses even though these results provide useful information to assist in such decision making. Other factors must also be considered, such as economics, environmental impact, safety, and social and political implications.