Introduction
Hypertrophic cardiomyopathy (HCM) is a complex cardiac disease with unique pathophysiological characteristics and a great diversity of morphological, functional, and clinical features. HCM is defined as primary myocardial hypertrophy in the absence of aortic valve disease or significant hypertension (Elliott, 2008)
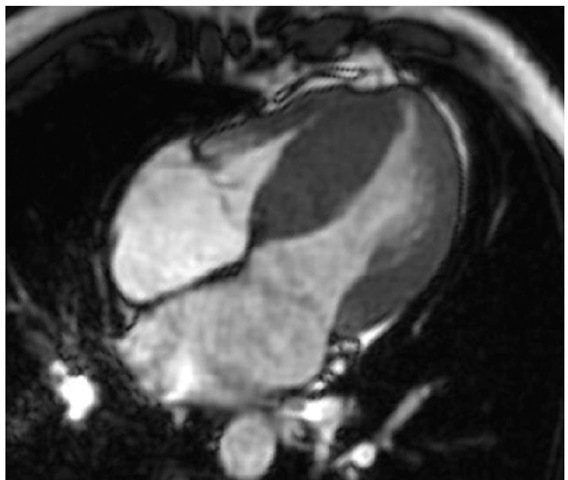
Fig. 1. Magnetic resonance imaging; hypertrophic left and right ventricle.
Several observations suggest that the prevalence of HCM is globally 1 in 500 (Maron, 1994). The condition therefore seems to be a common genetic malformation of the heart. The clinical course varies markedly. Some patients remain asymptomatic throughout their whole lives, some have severe symptomatology of heart failure or angina pectoris, while others die suddenly even in the absence of previous symptoms. The annual mortality rate varies in different studies. In unselected populations, it has been reported to be about 1% (Elliott, 2008). These observations suggest that a substantial proportion of patients with HCM has a more favourable course than previously believed. Two thirds of patients with HCM display evidence of obstruction in the left ventricular outflow tract (hypertrophic obstructive cardiomyopathy, HOCM), that is negatively associated with prognosis of the patients. During the last decade, technological developments in non-surgical treatment have provided new therapeutic options for patients with this disease. Recent changes have also generated considerable questions about the optimal management of HOCM and the role of percutaneous alcohol septal ablation (ASA).
With respect to various clinical courses, it seems to be impossible to define precise guidelines for management. As in many diseases, it is often necessary to individualise therapy.
Drug therapy of patients with hypertrophic obstructive cardiomyopathy
Drug therapy is used as the initial measure for controlling cardiac symptoms that has resulted in functional limitation. Beta-blockers and verapamil have traditionally been administered on an empirical basis, relying on the patient’s subjective perception of benefit. Drug selection is based on preferences of individual physicians. Most favour beta-blockers over verapamil for use in initial treatment, although it is not of critical importance which drug is used first. There is no evidence that beta-blockers or verapamil protect patients with HOCM from sudden death. On the other hand, they are able to relieve symptoms in the majority of patients. Whether these drugs should be used prophylactically to delay disease progression and improve prognosis in asymptomatic patients has been a subject of debate for many years. The effectiveness of a prophylactic treatment has not been tested prospectively because study populations are small and traditional endpoints are infrequent (death, clinical deterioration). Accordingly, we reserve medical therapy only for symptomatic patients.
Surgery to relieve the left ventricular obstruction
The group of patients who have both a large outflow gradient and severe symptoms and also do not respond to medical treatment are the best candidates for surgery. Based on outstanding experience of several surgical centres worldwide, myectomy has become the primary therapeutic option for patients with severe symptoms and marked functional disability (McCully, 1996). Surgical reduction of the outflow gradient is achieved by removing a small amount of muscle (5 to 10 g) from the basal septum. Recently, some surgeons have performed more extended myectomies and reconstruction of the subvalvular mitral apparatus. In these cases, the septal myectomy deeply extends into the left ventricular cavity. Subsequently, both papillary muscles are mobilised and all hypertrophied muscular trabeculae are also resected. Mitral valve replacement has also been used as an alternative therapy in selected patients, but the role of this operation remains unsettled. Surgery substantially reduces the basal outflow gradient in more than 90% of patients and provides large improvements in objective measures of symptoms and functional status.
However, the procedure requires extracorporeal circulation and great surgical experience. Mortality rates less than 2% are achieved only in heart centres with extensive surgical experience and numerous performed procedures. The effect of surgery on survival is not known. Accordingly, surgery is not performed in asymptomatic or mildly symptomatic patients.
Cardiac pacing to relieve the left ventricular obstruction
The role of cardiac pacing in reducing the left ventricular outflow gradient in HOCM was first reported more than 40 years ago (Gilgenkrantz, 1968). At the same time, it had been noticed that patients who developed left bundle branch block after septal myectomy had a better functional outcome. Sporadic reports over the years have culminated in the interest of cardiac pacing in the 1990s’.
In the early 1990s, several observational studies reported that dual-chamber pacing with shortened A-V delay was associated with both substantial decrease in the outflow gradient and symptomatic improvement of patients unresponsive to drug treatment. However, the mechanisms by which pacing might reduce outflow gradient was not understood. Later, three more carefully controlled and randomised studies found the effects of cardiac pacing to be less favourable (Linde, 1999; Maron, 1999; Nishimura, 1997). These studies were randomised, double-blind and crossover. Subjective symptomatic improvement was reported with similar frequency by patients after three months pacing and after the same period without pacing. Objective measurements of exercise capacity (for example maximal oxygen consumption) with and without pacing did not differ significantly. These findings suggested that a placebo effect might play an important role in the symptomatic improvement reported by the paced patients. Currently, cardiac pacing cannot be regarded as a primary treatment for patients with HOCM, although a modest reduction in outflow gradient is achieved in a number of paced patients. Probably a small subset of patients could profit from pacing, but this effect is inconsistent and usually only modest. Therefore, further randomized, controlled trials should be undertaken to resolve the uncertainties surrounding the utility and efficacy of cardiac pacing in patients with HOCM.
Alcohol septal ablation to relieve the left ventricular obstruction
The idea of inducing a septal infarction by catheter techniques was suggested by the observation that myocardial function of selected areas of the left ventricle can be suppressed by balloon occlusion of the supplying artery during angioplasty. The outflow pressure gradient in HOCM decreased significantly when the first septal artery was temporarily occluded by an angioplasty balloon catheter (Sigwart, 1982). This concept was also supported by observations that the outflow pressure gradient decreased after anterior myocardial infarction in HOCM patients.
Sigwart published his experience with "non-surgical myocardial reduction" of three patients with HOCM in 1995 (Sigwart, 1995). However, at the same time, other centres in Germany also started with the same interventional procedure (Kuhn, 2010). Since then, several modifications of the original technique have been described. The majority of authors prefer an echocardiography-guided anatomical approach for identifying the target septal branch,where the target septal branch is detected using myocardial contrast echocardiography (MCE). Estimation of the size of the septal vascular territory with MCE is accurate and safe. Using MCE, it is possible to delineate the perfusion bed of the septal perforators and predict the infarct size that follows the alcohol injection.
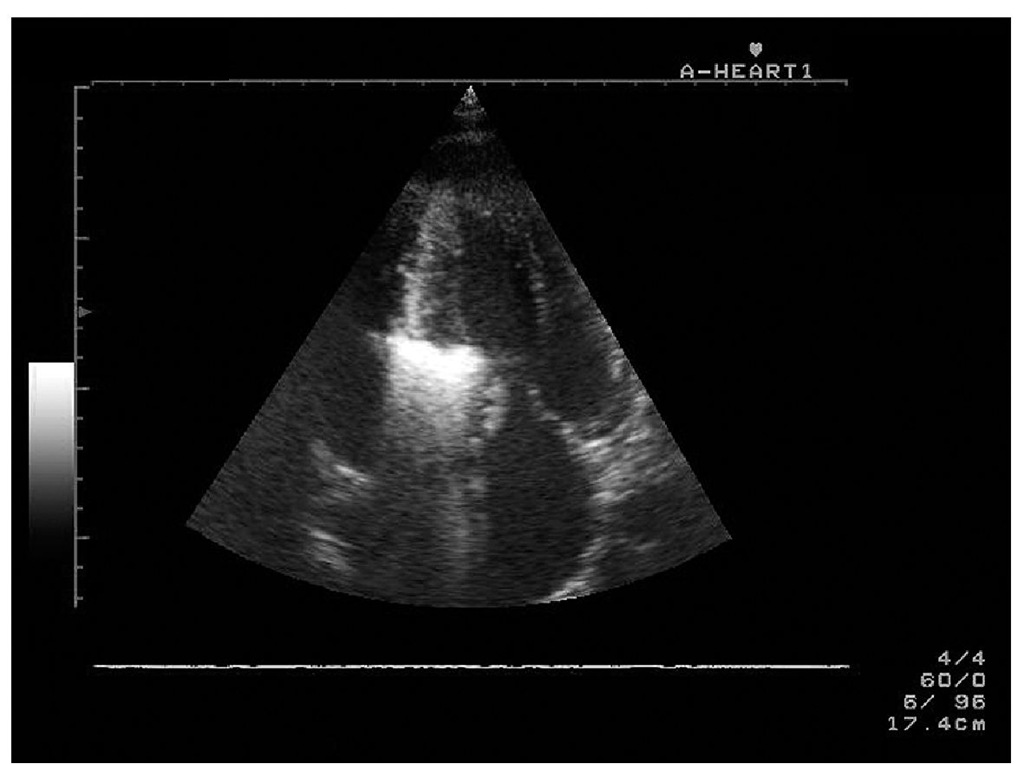
Fig. 2. Transthoracic echocardiography, apical 4-chamber view with optimal opacification of the basal interventricular septum by echocontrast medium.
Recently, we have proposed the use of real-time myocardial contrast echocardiography with very low mechanical index that allows better delineation of the target area than conventional contrast echocardiography.
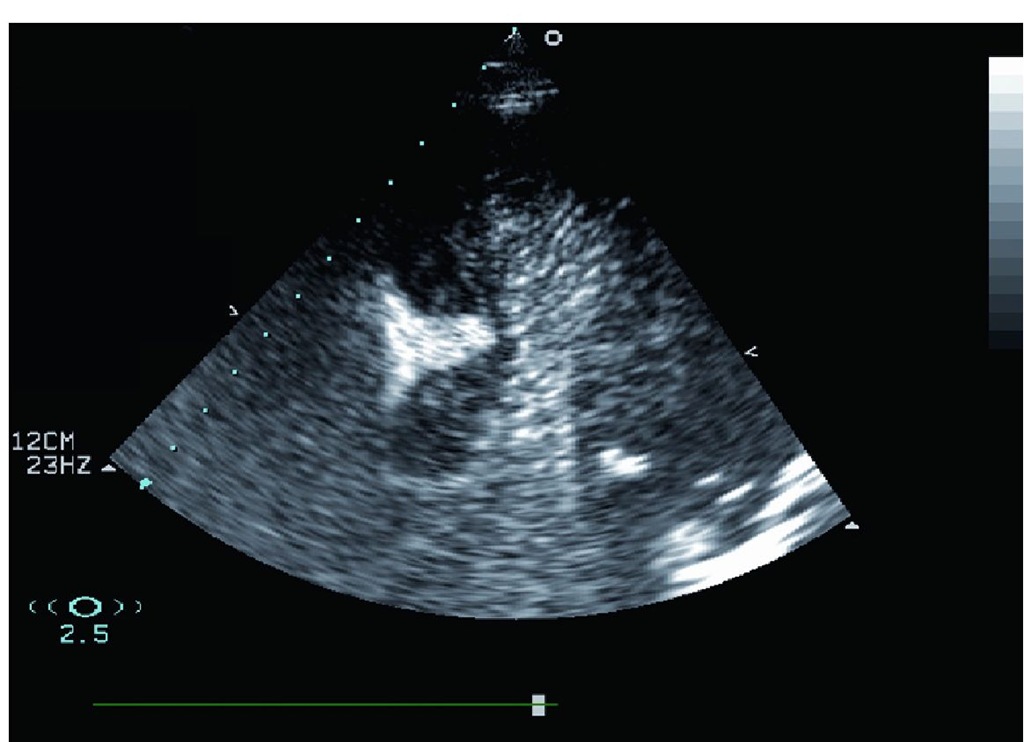
Fig. 3. Transthoracic echocardiography, apical 4-chamber view with real time myocardial contrast echocardiography utilizing power modulation and intracoronary injection of small amount of echocontrast agent.
There is a significant correlation between MCE septal area and reduction of ouflow gradient. In general, a higher biomarker level (extent of necrosis) was detected with larger sections of the infarcted septum. However, since alcohol injection is directed mainly to the portion of the septum causing the obstruction, many patients have a small defect with a large reduction of outflow gradient. Additionally, it has been found that MCE in 5 to 10% of cases shows contrast within myocardium away from the septal target area, indicating threatening misplacement of the myocardial necrosis. Accordingly, necrotisation of myocardium distant from the septal target area as a source of potentially fatal complications can be avoided by this approach. The introduction of echocardiographic guidance of ASA led to an improvement in haemodynamic results, despite a decrease in the infarcted septal area estimated by the maximal creatine kinase rise.
Indication
Accepted patient selection criteria for ASA were as follows: (1) anatomical findings of marked septal hypertrophy that projects from the LVOT; (2) dynamic obstruction of LVOT; (3) unresponsiveness to the medical therapy. There were no sufficient data available to confirm exact haemodynamic (pressure gradient > 50 mmHg), anatomical (septum thickness > 18 mm) or clinical (dyspnoea with NYHA class III or IV) criteria that resulted in a certain relaxation of indications in clinical practice. Patients with moderate symptoms were treated if they had high gradients and additional findings, such as recurrent exercise-induced syncope, markedly abnormal blood pressure response at exercise, paroxysmal atrial fibrillation or extremely high pressure gradient after provocative manoeuvres (Valsalva manoeuvre or use of nitrates). It is a very important question in clinical practice whether the increased risk of sudden cardiac death associated with LVOT obstruction justifies the use of ASA in slightly symptomatic or completely asymptomatic patients. At present, there are no sufficient data to answer this question. However, a relatively low risk of sudden death in asymptomatic patients with obstruction and none of the recognised risk factors for sudden death (1.ventricular tachycardia, 2. abnormal exercise blood pressure response, 3. family history of premature death, 4. unexplained syncope, 5. severe left ventricular hypertrophy in any myocardial segment > 30 mm) suggests that aggressive interventions are unjustified in this group. The situation in asymptomatic patients with obstruction and additional risk factors is less clear. The approach must be individualised. It seems to be reasonable to implant an AV-sequential implantable cardioverter-defibrillator (ICD) to try atrio-ventricular sequential pacing to reduce obstruction for 6 months, and then to perform ASA if needed. Nevertheless, ASA as the primary treatment is still unjustified in this group of patients, given potential mortality and morbidity associated with this procedure. Patient preference should, of course, be considered in such discussion and surgical myectomy should be mentioned as the gold standard in most western countries.
Alcohol septal ablation procedure
We usually recommend the following course of ASA procedure. A temporary pacemaker is placed in the apex of the right ventricle in everyone except patients who already have a permanent dual-chamber pacemaker in place. However, fluctuation of the pacing threshold has to be anticipated if the lead is directed towards the septum, i.e., with the tip in proximity to the ablation lesion. A multipurpose catheter is advanced through the aortic valve into the apex of the left ventricle and intraventricular gradient is measured by a pull-back technique. A 6 or 7F guiding catheter is then engaged into the ostium of the left coronary artery. Initial angiography is performed to localise the origin of septal arteries. Over-the-wire balloon catheter is introduced over a coronary wire into one of the major and proximal septal perforators and inflated. Contrast medium is injected through the central balloon lumen to delineate the area supplied by the septal branch and to ensure that balloon inflation prevents spillage into the left anterior descending artery. Contrast myocardial echocardiography is performed to delineate the area to be infarcted (Figures 1 and 2) and to exclude contrast (and subsequently alcohol) deposition in remote myocardial regions like the left ventricular posterior wall or papillary muscles. The optimal septal branch is identified by opacification of the area in the basal septum that is adjacent to the zone of maximal acceleration of the outflow jet and includes the point of coaptation between the septum and anterior mitral leaflet. Usually, the target septal branch originates from the proximal segment of the left anterior descending artery. However, in exceptional cases, it originates from diagonal or intermediate branches of the left coronary artery. Depending on the septal artery size and septal thickness, 1 to 2 ml of absolute ethanol are very slowly (2 to 3 minutes) instilled through the lumen of the inflated balloon catheter and left in place for 5 minutes. After balloon deflation and removal, angiography is done to confirm the patency of the left anterior descending artery and occlusion of the target septal branch. Measurement of intraventricular gradient is usually performed by a multipurpose catheter and guiding catheter and/or Doppler echocardiography. The gradient should decrease at least to one half.
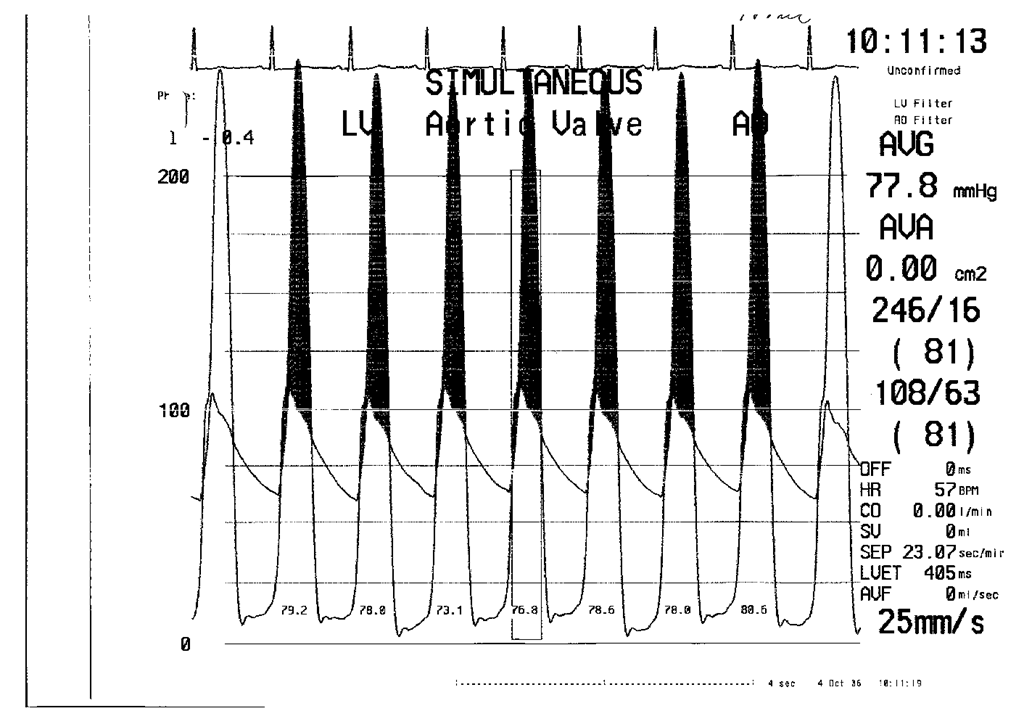
Fig. 4. Pressure gradient between left ventricle and aorta before ASA.
Fig. 5. Hemodynamic finding after ASA with nearly elimination of pressure gradient after procedure.
A temporary pacemaker is sutured in place. The patient is observed in the coronary care unit for at least 48 hours. If there is no high-degree atrioventricular block, the pacemaker lead is then removed.