Parasite tropisms
Geographic distribution of an organ-specific chronic disease (cardiac versus digestive diseases) and allocation of T. cruzi I and II (II to VI in the new classification), supported the hypothesis that disease outcome is linked to the T. cruzi genetic variations. Some studies did not show correlations among T. cruzi lineages and the clinical forms of Chagas disease (Zafra et al., 2011). Although, the presence of TcI was correlated with higher frequencies of electrocardiogram alterations than individuals infected with TcII, such as ventricular premature beats, first-degree atrioventricular block, sinus bradycardia, abnormal Q-waves, atrial fibrillation, and complex ventricular arrhythmias (Ramirez et al., 2010). In a mouse model infected with two different genetic populations of T. cruzi, both parasites were found during the acute infection in several host compartments (blood and organs). However, during chronic infection, a preferential tissue distribution with predominance of certain T. cruzi isolates was found (Andrade et al., 1999). Because T. cruzi mixed infections in triatomines are found in high rates, a similar phenomenon should take place during human infection.
Parasite invasion of the host cells
T. cruzi can reach the mammalian host cells via different mucosal tissues (i.e., conjunctiva, oral) or directly into blood (transfusion or congenital). The parasite in vivo can invade a vast range of cells such as monocyte/macrophages, dendritic cells, endothelial cells, fibroblasts, astrocytes, skeletal muscles, enteric nerves, and cardiomyocytes (Epting et al., 2010). Parasite invasion is a multistep process when several T. cruzi glycoproteins bind surface molecules on the host cells. Before reaching the target tissues, T. cruzi must interact with the endothelial cells to actively penetrate or increase endothelium vasodilatation. Parasite protease can produce inflammations that increase vascular permeability (Epting et al., 2010). Several parasite proteases and glycoprotein expressed by trypomastigotes have been associated with invasion: gp60 (penetrin) gp63, gp35/50, gp82, gp90 a parasite glycosidase, mucins and transialidase such as gp85 or Tc85. The binding of these parasite molecules to host molecules (cytokeratin 18, mucins, heparan sulfates, extracellular matrix proteins such as fibronectin and laminin, and carbohydrates with sialic acid) induce Ca++ mobilization, protein tyrosine phosphorylation and cytoskeleton reorganization in the target cells. Transialidase, glycosylphosphatidylinositol (GPI) anchors surface-bound proteins are in charge of transferring sialic acid residues from the host cell to the parasite glycoproteins. This mechanism seems to be crucial in invasion given that trypomastigotes with no expression of trans-sialidases were poorly invasive to non-phagocytic cells (Epting et al.,2010). Also, infection by oral route involved other parasite glycoproteins such as mucin-like gp35/50 or gp82 on the surface of the trypomastigotes, resistant to protease digestion. Glycoprotein gp82 binds to the gastric mucin and allows the parasite to invade epithelial cells (Yoshida, 2008).
Acute and chronic heart involvement in Chagas diseases
Acute myocarditis
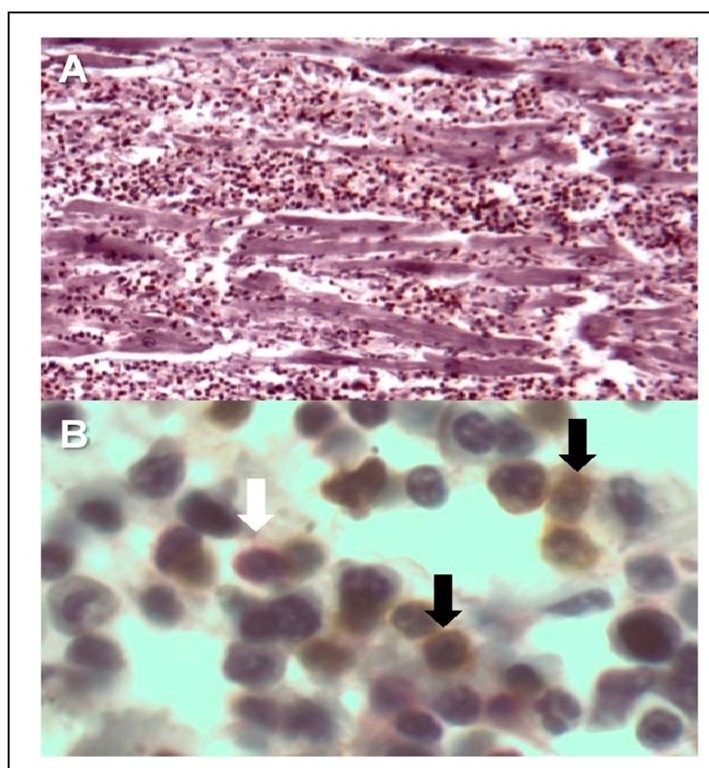
Parasite genetic variations, the initial parasite burden, and the host immune response seem to influence the evolution of the Chagas disease. Clinical cardiac involvement is found in nearly 90% of symptomatic patients. Parasite-infected myocardiocytes with intracellular amastigotes (pseudocyst) break up and induce acute inflammation (Coura & Borges-Pereira, 2010). There is massive and diffuse infiltration with predominance of mononuclear cells (Fig. 3A) mainly CD8+ T lymphocytes (LT) up to 60% (Fig. 3B). Whether these LT are T. cruzi antigen specific and which chemotactic agents control this migration is unknown. Microarray studies of T. cruzi acute infection in mouse cardiomyocytes showed a regulation of 353 genes (111 up regulated and 242 down regulated) associated with inflammation, cytoskeleton, cell interaction, apoptosis, cell cycle, and oxidative stress. Interestingly, early genes up regulated include a vast range of chemokines, which attract mononuclear cells (Manque et al., 2011). In consequence, there is cell destruction (myocytolysis), interstitial edema, hypertrophy of myocardial fibers, and alteration of the cardiac microcirculation with platelet aggregation, production of pro-inflammatory cytokines and expression of vascular adhesion molecules by endothelial cells (Rossi et al., 2010).
Fig. 3. Immunohistology of a heart with acute Chagas disease showing extensive cellular infiltration 10x (A), and presence of CD8+ (black arrow and brown cells) and CD4+ T cells (white arrow and red cell) with hematoxylin as contra-staining 100x (B).
Chronic myocarditis
Contrary to acute infection, in the chronic phase there is scarcity of T. cruzi niches; however, there is an extensive, but patchy mononuclear infiltration, with predominance of macrophages and cytotoxic CD8+ T cells (CTL). Myocarditis has a slow progression with changes in the contractile function and dilatation of the heart walls. Increase in metalloproteinase has been described in infected cardiac tissue and associated with remodeling of the extracellular matrix (Gutierrez et al., 2009). Histology analysis shows diffuse myocarditis, myocytolysis, edema, mononuclear cellular infiltration (hallmark of the delayed hypersensitivity), destruction of the conduction system with neuron loss (autonomic denervation), and extensive myocardial fibrosis. Functional studies in Chagasic cardiopathy demonstrated impaired perfusion at the coronary vessels due to microvascular changes (thrombi, inflammation, and spam) (Rossi et al., 2010).
Mechanisms of tissue damage
Autoimmunity
The autoimmune theory was initially based on the scarcity of parasites found in chronically infected tissue and also on the presence of antibodies and T cells that recognized parasite antigen and cross-reacted with host tissues and molecules. Antibodies against T. cruzi bind to human laminin, sulfo-galactosylceramides, cardiac myosin, microtubule-associated proteins, ribosomal proteins, P-adrenergic and muscarinic receptors; heart sarcolemma, blood vessel, neurons, glial cells, myocardium and skeletal muscles (Bonney & Engman, 2008). However, demonstration of the pathological consequences due to autoimmunity in T. cruzi infection does not have direct evidence. Most of the auto-antibodies are considered to be natural antibodies that could be induced after tissue injury and exposure of host cell molecules. Also, T. cruzi antigens can act as B cell polyclonal stimulators. Against the autoimmunity theory it is known that the immune-suppression exacerbates T. cruzi infection and specific anti-parasitic treatment ameliorates the clinical disease (Rossi et al., 2010).
Antigenic persistence and immune response
By using DNA techniques, the presence of T. cruzi in tissues during chronic infection has become clear. Antigen persistence triggers inflammation and lymphocyte infiltration. Damage mechanisms are unclear because parasite burden does not explain extensive cell loss. CD8+ T lymphocytes contribute to cytotoxicity probably via perforin and granzyme B, and TGB-P and interleukin-10 (IL-10) secreting macrophages can induce repair and fibrosis through fibroblasts. T. cruzi infection also alters microcirculation with the presence of platelets aggregated, microvascular spam, and secretion of vasoconstrictor agents such as tromboxane A2 (TXA2) and platelet activated factors (PAF) by macrophages or endothelin 1 (ET-1) by endothelial cells (Rossi et al., 2010).
Human immune response
Innate immune cells such as natural killer cells, macrophages, and dendritic cells detect invading pathogens and alert the immune system through activation cascades. The aim is to elicit innate antimicrobial and inflammatory responses and initiate adaptive immunity to control or eliminate infection. It is accepted that the establishment of chronic infection with T. cruzi is a consequence of the inability of the immune response to elicit sterilizing anti-parasite immunity. Therefore, the host innate and adaptive immune response is believed to be the key determinant of the clinical outcome of the disease.
Innate immunity
Dendritic cells (DCs), natural killer (NK) cells, and monocytes are vital mediators of the innate immune system and promote development of adaptive immune responses. Evidence shows that T. cruzi may infect DCs and even proliferate inside them. Consequently, the DC antigen presentation capacity is reduced (Van Overtvelt et al., 2002). In early asymptomatic Chagas disease, higher levels of pro-inflammatory monocytes and expansion of NK cells before the adaptative immunity development has been shown (Vitelli-Avelar, 2006). The role of cytokines such as interleukin (IL)-4, IL-12, TNNFa, and interferon (IFN)y secreted by these cells can be an important element for host resistance during the early stages of infection and also in the genesis of myocarditis (Golgher et al., 2004). It has been shown that two different and independent antigenic stimuli from the parasite induce both an enhancement of IL-10 and a reduction of IL-12 secretion in DCs from Chagasic patients compared to DCs from healthy donors (Cuellar et al., 2008). Although, the innate immune system seems to have a fundamental role in Chagas disease by controlling parasite replication and spread in host tissues, it is not clear if events described here, that mediate inflammatory reaction, can be related to protection or tissue damage in the chronic phase of the disease.
Humoral immune response
A specific antibody response and B cells in animal models of Chagas disease seem to play an important role for parasite control, especially against the trypomastigotes. In spite of the large number of parasite proteins some molecules have been studied. Indeed, in our previous work, we showed that there is a consistently higher specific IgG response in chronic Chagasic patients against T. cruzi kinetoplastid membrane protein-11 (KMP-11), and the T. cruzi heat shock protein-70 (HSP-70). The recombinant KMP-11 protein recognition was focused on IgG1 sub-fraction; whereas, the lysate was on IgG3 plus IgG1 in asymptomatic and cardiopathic chronic phases, compared to acute sera from Chagasic patients (Flechas et al., 2009). These data reflect the dynamics of the humoral immune response in Chagas disease and may be an important issue given that IgG1 and IgG3 are the major complement fixing isotypes, which also mediate cooperative function with phagocytes; nevertheless, the role of these specific antibodies in controlling the infection or progressing in disease severity need to be addressed.
T cells and cytokines
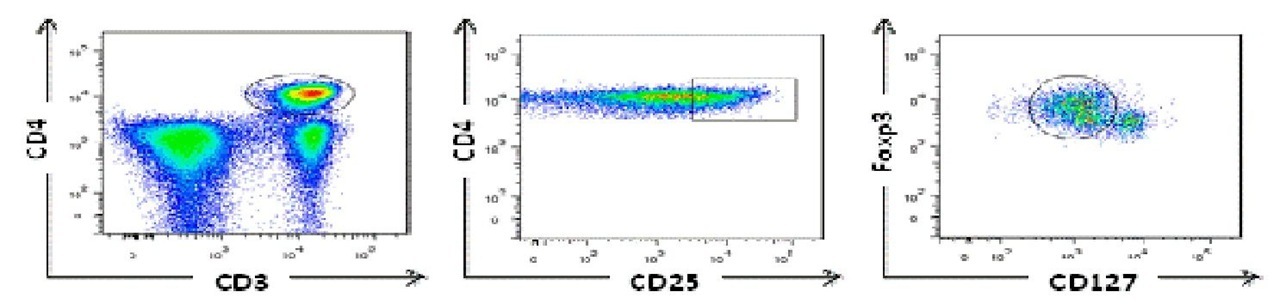
Individuals undergoing chemotherapy generally show protection against viral infections controlled by T cells during lymphopenia, indicating that a small population of T cells can be protective (Turtle et al., 2009). However, reactivation of Chagas disease, defined by a demonstration of trypomastigotes on microscopic examination of blood or the identification of amastigotes on biopsy samples and/or acute clinical manifestations during the chronic phase, can occur among the immunosuppressed patients with heart transplantation (Burgos et al., 2010) or AIDS patients (Almeida et al., 2009). It may be the natural history disease demonstration that T cell response is crucial to control parasite burden and clinical manifestations in a large proportion of patients. Perhaps the most interesting question is how adaptive immune response can contribute to most infected individuals remains asymptomatic whereas an important percentage of these patients develop severe forms of the disease. In humans, it has been shown that CD4+ T cells (Cuellar et al., 2009) and CD8+ T cells (Fiuza et al., 2009) from Chagasic patients specifically produced IFNy after exposure to T. cruzi antigens. Furthermore, chronic Chagasic patients had lower levels of antigen-specific CD8+ T cells secreting IFNy compared with non-symptomatic individuals (Laucella et al., 2004). Because T. cruzi is an intracellular parasite, many groups have focused on the study of CD8+ T cells. Some of them have studied specific CD8+ T cells against peptides derived from cruzipain, FL-160 (Fonseca et al., 2005), KMP-11 (Diez et al., 2006; Lasso et al., 2010), and trans-sialidases (Alvarez et al., 2008) proteins, founding similar frequency of specific CD8+ T cells for these epitopes. Nonetheless, it has been shown that patients with more severe forms of Chagas disease have more differentiated CD8+ T cells which could have lost their functional capacity (Bixby & Tarleton, 2008). One interesting aspect is the control of immune response by regulatory T cells (Treg). Ex vivo, it was shown that children with asymptomatic Chagas disease display a lower frequency of natural Treg CD4+ CD25high compared to non-infected children (Vitelli-Avelar et al., 2006). Interestingly, these cells are in increased levels in peripheral blood of late chronic asymptomatic patients (Vitelli-Avelar et al., 2005). These data suggest that Treg could be important to limiting tissue damage. However, taking into account that additional molecules have been suggested to identify Treg, we used a panel of antibodies for CD4, CD25, FoxP3, and CD127. Our results show higher proportion of Treg in symptomatic chronic Chagasic patients compared to non-infected individuals, indicating that the frequency of Treg can contribute to damage. Fig. 4 despites the CD4+ Treg cells by flow citometry (Lasso et al., 2009).
Fig. 4. Regulatory T cells from chronic Chagasic patient identified by high levels of expression of the transcription factor forkhead box transcription factor P3 (FoxP3) and low levels of CD127.
Diagnosis
The diagnosis of Chagas disease, as with other infections, is performed on the basis of clinical findings, parasite presence, serological status, and epidemiological data. Furthermore, the disease stage is also an important fact to consider. For instance, as the parasitemia dramatically decreases from acute to chronic phase, in the early phase parasite detection is achieved by parasitological conventional direct tests (see below). Nevertheless, because clinical findings in this stage can be confused with other pathologies, the epidemiological data demonstrating a connection between the patient and the parasite is of special importance (Nicholls et al., 2007). In contrast, in chronic patients, the presence of symptoms or abnormal clinical findings usually correlates with the disease but parasite concentration is low and variable. Bearing in mind that T. cruzi infection is life lasting; in the chronic phase serological tests are applied to indirectly demonstrate parasite presence (Enciso et al., 2004). Indeed, the WHO recommends that to diagnose a chronic Chagasic patient; besides having clinical findings compatible with Chagas disease and history of vector contact, there must be at least two positive serological tests with different immunological principles. Finally, chronic asymptomatic patients represent a real challenge for diagnosing inasmuch as there are no clinical findings, and again parasitemia is very low and intermittent. Consequently, the epidemiological patient history is also of most importance (Gil et al., 2007).
Clinical findings
Electrocardiogram
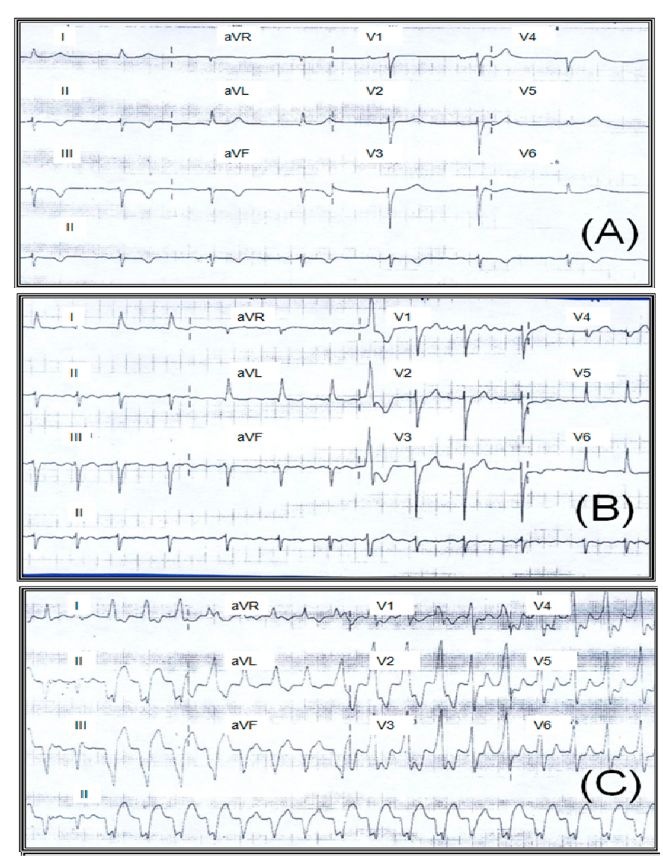
The most common electrocardiographic manifestations are right bundle branch block (RBBB), anterior fascicular block, premature ventricular contractions, changes in ST segment and T wave, abnormal Q waves, and low voltage of the QRS complex (Fig. 5). The combination of the RBBB and the anterior fascicular block suggest the disease (Garzon et al., 1995). The presence of frequent premature ventricular contractions, including duplets and salvos of non-sustained ventricular tachycardia are a common finding in the Holter monitoring and in the stress test. Premature ventricular contractions correlate with the severity of the ventricular function, but can also occur in patients with preserved ventricular function. Episodes of non-sustained ventricular tachycardia are observed in 40% of the patients with light to moderate ventricular contractibility alterations and in virtually all patients with heart failure, which is more frequent than in other cardiomyopathies. Sustained ventricular tachycardia is another disease marker. This arrhythmia can be produced through programmed ventricular stimulation in nearly 85% of the cases and results from intramyocardial or subepicardial reentrant phenomena, usually located on the inferoposterior and lateral wall of the left ventricle.
X-ray and echocardiography
In patients in the undetermined phase, the cardiac silhouette evaluated in the chest X-ray and the global systolic function in echocardiography are normal. In more advanced stages, the chest X-ray can show cardiomegaly and pulmonary congestion. The disease can cause diffuse damage of the systolic function of the left ventricle. The global systolic function of the left ventricle has prognostic implications. In a cohort of 538 patients grouped into four stages of disease progression, different survival rates were found in the five-year follow up from 98%, 91%, 45% to 13% for those with normal left ventricle function, moderately depressed function, with reversible heart failure, or irreversible heart failure, respectively (Rassi et al., 2010). Some alterations of the segmental contraction of the left ventricle can be detected. The most common is located on the posterior wall with 20% prevalence. The presence of mitral or tricuspid insufficiency is generally associated to ring expansion. The prevalence of aneurysms in the left ventricle varies in the different series, noted on an average of 8.5% in asymptomatic individuals and in patients with severe cardiac damage up to 55%. Through logistic regression analysis, the presence of an apical aneurysm in the left ventricle was an independent predictor of mural Thrombi (Albanesi-Filho et al., 1991). In another work, the finding of an aneurysm was significantly associated to a thrombus and cerebro-vascular accident during a two-year follow up. On some instances, diminished systolic function of the right ventricle can be the only abnormality detected via echocardiography; in general, it is secondary to the severity to the damage of the left ventricle and at high levels of pulmonary pressure. With regards to diastolic function, chronic myocarditis in Chagas disease can diminish ventricular relaxation and diastolic filling. These abnormalities usually precede systolic dysfunction. Reduced compliance of the left ventricle can increase the filling pressure of the left atrium with changes in transmitral and pulmonary venous flow rates. The echocardiography study is recommended as a routine clinical evaluation method in patients with Chagas cardiopathy to determine the stage of the disease, its progression, as well as to estimate survival, dismiss the presence of aneurysms or intracavitary thrombi, and monitor response to treatment.
Fig. 5. ECG sequence in a 72-year-old woman diagnosed with Chagas cardiomyopathy and ejection fraction of the left ventricle at 25%. Note sinus bradycardia (A), attrial fibrillation (B), and monomorphic sustained ventricular tachycardia (C). Fundacion Cllnica Abood Shaio, Bogota D.C., Colombia.
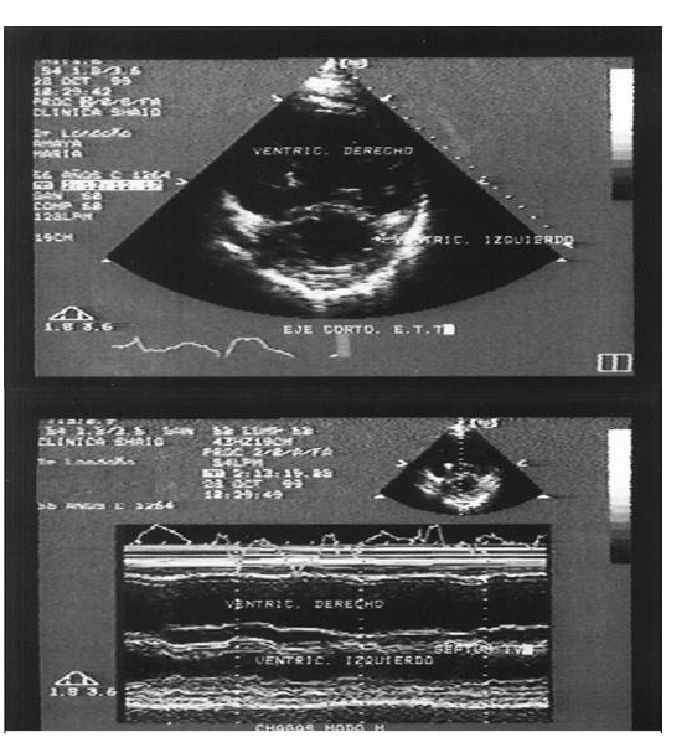
In our experience at Fundacion Cllnica Abood Sahio (Bogota, Colombia), from a total of 120 patients evaluated with diagnosis of Chagas cardiomyopathy, 73 women (60%) with mean age of 56.7 +/- 13 years (21-84), clinical manifestations corresponded to dyspnea (42%), palpitations (31%), chest pain (42%), presyncope (24%), syncope (27%), and aborted sudden death (2.5%). Nearly 6.7% of the cases did not present clinical manifestations. The main ECG findings were: right bundle branch block (40%), second and third degree AV block (29.2%), dysfunction of the sinus node (28.3%), ventricular tachycardia (23%), atrial fibrillation (19%), left anterior hemiblock (17.2%), atrial flutter (3.3%), and left bundle branch block (3.3%). In 31% of the cases, the chest X-ray was normal. In 15.8%, severe cardiomegaly was observed. All the patients were subjected to a color Doppler echocardiogram according to internationally recognized norms, finding a mean fraction of the left ventricle of 43.3% (SD +/- 16.5) (10-60) and of the right ventricle at 23.4% (10-40) (Fig. 6). The study was considered normal in 33.6% of the cases. Contractility alterations were documented in 42.4%, with these being globally in 26.5% of the cases, or inferior, apical-inferior and anterior localization. Isolated compromise of the right ventricle was observed in one case (0.8%), suggesting the diagnosis of arrhythmogenic dysplasia of the right ventricle. In 24% of the cases mitral insufficiency was evidenced and 15.2% revealed tricuspid insufficiency. A total of 11 aneurysms (9.7%) were observed, 63.6% of apical localization and 36.3% of inferior localization. Some 8.8% of the patients presented intracavitary thrombi, generally related to aneurysms or global contractility alterations. Holter or electrophysiological study documented ventricular tachycardia (sustained or unsustained) in 19.4% of the cases. Additionally in 10% we observed association to sinus dysfunction and/or AV block with ventricular tachycardia. Anatomic-pathological findings obtained via biopsy or surgery in 10 Chagas patients were: a) hypertrophy and/or b) fibrosis and/or c) chronic inflammatory infiltrate. None of the cases reported parasites in the samples examined by pathology (Rosas et al., 2007).
Fig. 6. Echocardiography M mode (A) and bi-dimensional (B) of 54-year-old female with a history of aborted sudden death due to ventricular tachycardia) secondary to Chagasic cardiomyopathy. Note the severe dilatation of right ventricle.