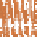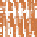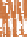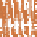Geoscience Reference
In-Depth Information
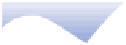
Initial uplift
Eroded mass
Mean sea level
Sediment
Buoyant lithosphere
Isostatic
uplift
Loading
Denser
asthenosphere
(a)
Simplified illustration of isostasy with wood blocks
floating in water.
(b)
Orogenic uplift, erosion, sediment transfer and
isostatic adjustment.
Ice sheet
Fore-bulge
Eustatic rise
Eustatic
fall
SL
1
SL
2
SL
2
SL
1
Isostatic rebound
Loading
Flexural isostasy
Flexural isostasy
Crustal depression
(c)
Glaciation, crustal depression, complex margin
responses and eustatic sea-level fall.
(d)
Deglaciation, isostatic rebound, sediment
transfer and eustatic sea-level rise.
marine biosphere and sediment source. Exceptions occur
at particular points of major
influx
or
efflux
of water
and/or minerals such as the ocean-atmosphere boundary,
estuaries, tidewater glaciers and human pollution sources.
We note that increased fresh water or decreased mineral
flux
dilutes
and decreased fresh water or increased mineral
flux
concentrates
the solution. Density varies inversely
with temperature but is complicated by changes in salinity,
outlined below. In addition to mineral solutions and
suspensions, oceans are also reservoirs of dissolved
atmospheric gases, incorporated by diffusion from the
atmosphere and in sea spray. Concentrations are usually
related directly to pressure and inversely to temperature.
Average concentrations of N
2
and O
2
are about 1·1 parts
and 0·5 parts per thousand respectively, but at 1·3 parts
per thousand CO
2
is much more abundant, given its low
atmospheric mass. Levels of both oxygen and carbon
dioxide vary considerably in the photic zone (see below)
as a result of biosynthesis.
OCEAN WATER
Ocean water chemistry
Ocean water is a weak cocktail of nearly 90 per cent of
known elements, dissolved in 1·4 G km
3
of sea water or
carried in suspension largely from terrigenous sources.
Most elements occur only as traces (less than one part per
million) and just eleven account for over 99 per cent of
solutes - Cl, Na, Mg, K, Ca, Si, Cu, Zn, Co, Mn and Fe, in
order of mass. This is fairly similar to the compositional
character of the lithosphere. The elements are derived
from continental crust erosion, sea water/crust interac-
tions and direct rainfall. They raise water density from 1·0
gm cm
-3
to an average of 1·03 gm cm
-3
with an alkaline
pH of 7·8-8·4. The vast bulk of ocean water is chemically
homogeneous and stable, despite substantial active fluxes
of water and minerals between adjacent 'spheres' and