Geoscience Reference
In-Depth Information
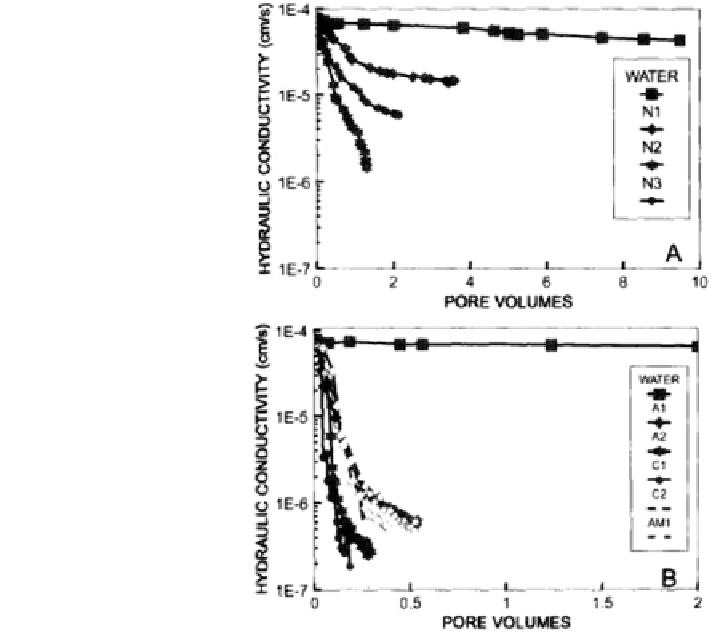
Fig. 18.32 Hydraulic
conductivity versus pore
volume (1 mol/kg solution
concentration) for a nonionic
surfactants, and b ionic
surfactants. N1-3 nonionic;
A1,2 anionic; C1.2 cationic;
AM1-amphoteric surfactants.
Reprinted from Allred and
Brown (
1994
). Copyright
1994 with permission of John
Wiley and Sons
Surfactant-induced deflocculation and dispersion of clay particles into the soil-
subsurface matrix are other factors leading to irreversible changes in the soil
hydraulic conductivity. The application of nonionic surfactants either before or
during irrigation was found to increase the dispersion of hydrophobic sandy soils
(Mustafa and Letey
1969
), leading to a decreased flow rate (Miller et al.
1975
).
Sodium dodecylbenzenesulfonate (SDBS) is a major constituent of synthetic
detergents used in household products and industrial processes; it has been found
in municipal sewage waters. Rao et al. (
2006
) used SDBS in a laboratory exper-
iment to test the effect of anionic surfactants on dispersivity and hydraulic con-
ductivity of sodium and calcium-saturated silt loam paddy soil. Dispersion of
Na- and Ca-soil with SDBS in NaCl solutions was determined by measuring the
relative absorbance (A
R
) of the suspensions. Relative absorbance is defined as
A
R
= A
x
/A
0
, where A
x
and A
0
are the absorbance of soil suspensions at increasing
SDBS and NaCl concentrations, respectively.
The A
R
of the suspension in the SDBS solution increased with SDBS con-
centration, indicating an increase in the stability of the suspensions (Fig.
18.33
).
To explain this behavior, Rao et al. (
2006
) suggested the following mechanism: (1)
Adsorption of SDBS on the clay particles, which increases the repulsive forces and