Geoscience Reference
In-Depth Information
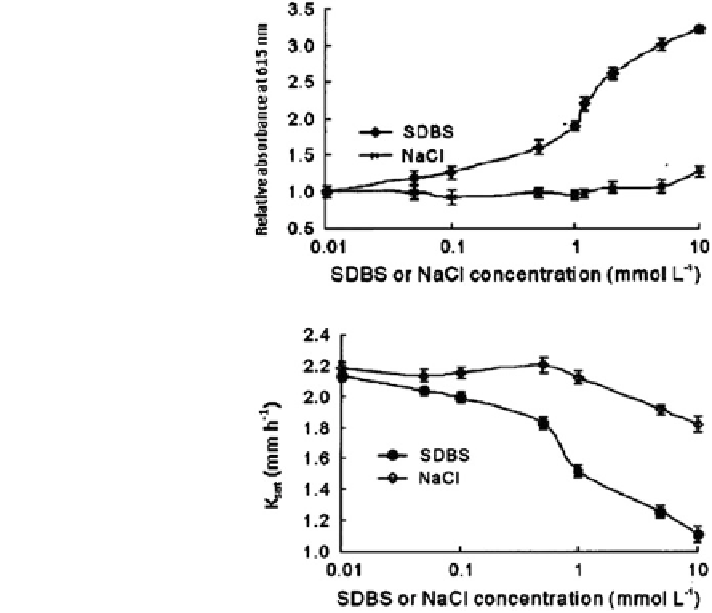
Fig. 18.33 Effect of sodium
dodecylbenzenesulfonate
(SDBS) and NaCl on the
stability of soils saturated
with calcium at 25 ± 1C
measured by relative
absorbance (modified after
Rao et al.
2006
) Reprinted
from Rao Rao et al. (
2006
).
Copyright 2006 with
permission from Elsevier
Fig. 18.34 Variations in
saturated hydraulic
conductivity, K
sat
, with
concentration of sodium
dodecylbenzenesulfonate
(SDBS) and NaCl injected
into soils saturated with
calcium at 25 ± 1C
(modified after Rao et al.
2006
) Reprinted from Rao
et al. (
2006
). Copyright 2006
with permission from
Elsevier
causes dispersion of particles; (2) Adsorbed SDBS tends to decrease interface
tension, supporting particle dispersion; and (3) increase of Na
+
in the Ca-soil
suspension, promoting particle dispersion. Combination of these three processes
results in an increase in the stability of the Ca-soil suspension. In contrast, the A
R
of the suspension in NaCl solution varied little below 1.0 mmol/L NaCl. Above
this value, the A
R
increased slowly.
The dispersion of clay particles in Ca-soil columns leached by SDBS and NaCl-
aqueous solution led to an irreversible decrease in soil hydraulic conductivity
(Fig.
18.34
). The higher the SDBS or NaCl concentration in the flushing solution,
the greater the extent of the hydraulic conductivity decrease. However, as in the
dispersion experiments, the effect of SDBS was much greater than that of NaCl.
These results show that the hydraulic conductivity of smectite clay-sand mix-
tures decreased following addition of various anions. Dispersed clay, however,
appeared in the effluent only upon addition of citrate or hexametaphosphate
(Frenkel et al.
1992
). In the latter case, the hydraulic conductivity began to
increase once maximum clay concentration reached the effluent. Dispersion of
smectite was one order of magnitude lower than that of kaolinite; illite exhibits an
intermediate value. Frenkel et al. (
1992
) attributed this behavior to kaolinite
having the highest ratio of positively charged edge surfaces to negatively charged