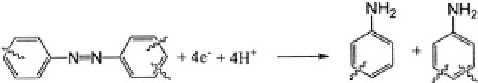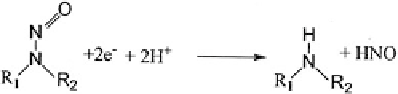Geoscience Reference
In-Depth Information
Table 13.4 Reductive transformation known to occur in natural reducing environments (Larson
and Weber
1994
)
1. Reductive dehalogenation:
Hydrogenolysis:
Vicinal dehalogenation:
2. Nitroaromatic reduction:
3. Aromatic azo reduction:
4. Sulfoxide reduction:
5. N-nitrosoamine reduction:
6. Quinone reduction:
7. Reductive dealkylation:
Nitroaromatic Reduction Nitroaromatics constitute an important class of
potential environmental contaminants, because of their wide use in agrochemicals,
textile dyes, munitions, and other classes of industrial chemicals. Reduction of
nitroaromatics produces amines, through a series of electron transfer reactions with
nitroso and hydroxylamines as intermediates (Fig.
13.1
). Compared to the parent
nitroaromatic compound, all intermediates typically reduce readily (Larson and
Weber
1994
).
Aromatic Azo Compounds Reduction of aromatic azo compounds involves a
four-electron process that proceeds through a short-lived intermediate, hydrazo-
benzene, and ends with complete reductive cleavage of the azo linkage and for-
mation of aromatic amines.
Sulfoxide Reduction Sulfoxide reduction is a two-electron-transfer reversible
reaction resulting in thioethers. Organic sulfoxides are used mainly as agro-
chemicals, and their reduction (abiotic and microbially mediated) has been found
in anaerobic soils, sediments, and groundwater (Larson and Weber
1994
).