Geoscience Reference
In-Depth Information
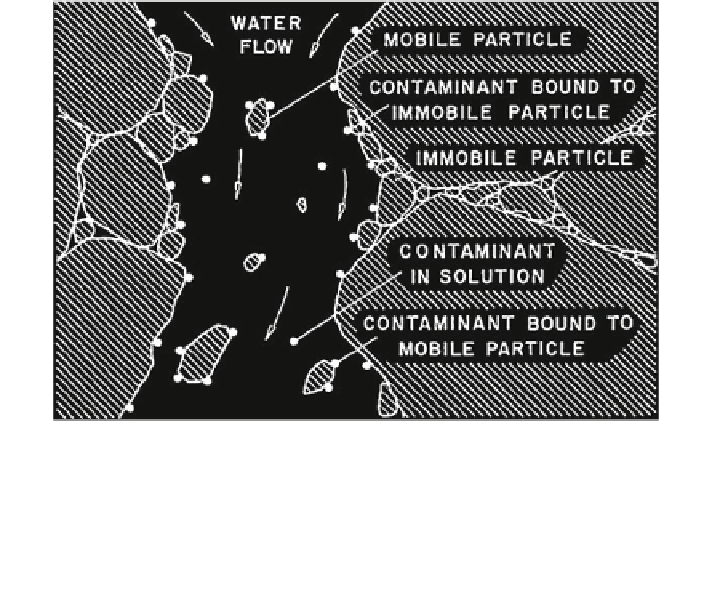
Fig. 12.2 Schematic representation of colloid-facilitated contaminant transport within a pore of
a water-saturated porous medium in the subsurface. Contaminants (filled circle) are either
dissolved in the liquid phase or adsorbed to the surfaces of the solid phase. The entire particular
phase is commonly assumed to be at rest, but it is possible that colloidal particles disperse in the
liquid phase and provide a rapid transport pathway for the contaminant (Grolimund et al.
1996
).
Reprinted with permission from Grolimund et al. (
1996
). Copyright 1996 American Chemical
Society
affinity of both heavy metals increases with organic carbon, surface area, and pH
of the colloid fraction, and is greater for Cu than for Zn.
Colloid-mediated transport of Cu and Zn was established by eluting the con-
taminants disposed on two soil columns (Loradale and Maury) with an aqueous
solution. Figures
12.4
and
12.5
show breakthrough curves for Cu and Zn eluted in
the absence and in the presence of colloids. The presence of colloids enhanced
metal transport through the soil columns, with the total eluted heavy metal
(colloid-bound ? soluble) being greater than that of the control treatment (heavy
metal without colloid). The colloid-mediated metal transport was as high as 50
times that of the control. Karathanasis (
1999
) notes the effect of soil composition
and structure, and of the properties of the heavy metals themselves, on their
colloid-enhanced transport. The author suggests that a stronger metal affinity for
colloid surfaces than for the soil matrix appears to be the dominant mechanism
facilitating heavy metal transport. However, physical exclusion, competitive
adsorption, and metal solubility are additional factors contributing to the extent of
colloid-mediated transport.
Soil colloid release and subsequent aqueous transport are strongly affected by
the solution pH; as such, the extent of facilitated transport of an adsorbed heavy
metal is pH dependent. A particular case of facilitating Pb release from a con-
taminated podzolic acid soil by increasing pH is reported by Klitzke et al. (
2008
),