Geoscience Reference
In-Depth Information
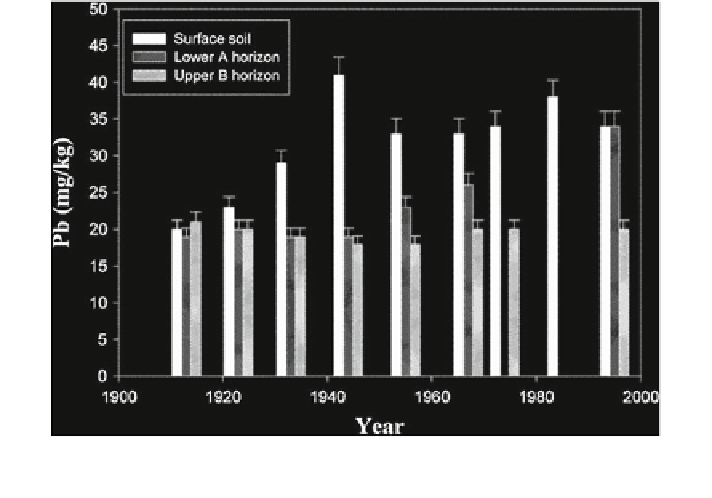
Fig. 12.1 Lead contents (mg/kg) in surface and subsurface soils during 100 year of cultivation
(Zhang
2003
)
between 1 lm and 1 nm in size, colloids in the subsurface may be of mineral,
organic, or biological origin. Colloidal fractions in the soil-subsurface solid phase
may interact with heavy metals, inducing an increase in their mobility. A labo-
ratory experiment by Grolimund et al. (
1996
) examined colloid-facilitated aqueous
transport of lead in a silty loam soil column and developed a schematic repre-
sentation of colloid-facilitated contaminant transport (Fig
12.2
). The contaminant
is transported either dissolved in the liquid phase or as adsorbed to the surface of
the solid phase.
The decrease in ionic strength may favor the condition for enhanced soil colloid
release and transport. Figure
12.3
shows the experimental results. The soil col-
umns were first saturated with Na
+
and contaminated with PbCl
2
. Under these
conditions, Pb was strongly adsorbed on the soil. Subsequently, leaching the
column with a CaCl
2
solution led to a decrease in the ionic strength, and two fronts
moving through the porous media were observed. Colloid release occurs between
these two fronts, associated with a significant release of a colloid-bound Pb. The
transport of colloid-bound Pb increased, confirming the pathway of heavy metal
and metalloid colloid-facilitated transport.
An example of colloid-facilitated water transport of Cu and Zn is given by
Karathanasis (
1999
). The transport of heavy metals adsorbed on colloidal fractions
of six soils from Kentucky, USA, through undisturbed soil columns was studied, in
comparison with the transport of dissolved compounds. Properties of the soils and
soil colloids used in the experiment are reproduced in Table
12.2
. The sorption