Geoscience Reference
In-Depth Information
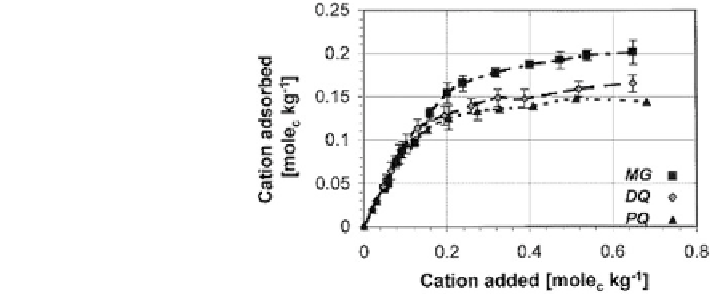
Fig. 8.28 Adsorption
isotherms of methyl green
(MG), diquat (DQ), and
paraquat (PQ) on sepiolite.
Reprinted from Rytwo et al.
(
2002
). Copyright 2002 with
permission of Elsevier
vermiculite, illite, and kaolinite. We see from these data that the cations can have a
significant influence on the extent and energy of the adsorption processes.
Exchange of the resident cations by both of the organo-cations was essentially
complete in the case of kaolinite, montmorillonite, and illite clays, although there
was a slight preference for paraquat over diquat in homoionic montmorillonite.
The affinities were reversed in the case of adsorption by Na
+
-vermiculite and illite
clays.
Competitive adsorption between the organo-cationic herbicides diquat and
paraquat and salts or a monovalent organic compound also was considered by
Kookana and Aylmore (
1993
). An increase in the salt concentration of the soil
solution from 0.005 to 0.05 M CaCl
2
resulted in decreases in sorption capacities
for the studied herbicides.
The effect of NaCl concentration on the rate of paraquat adsorption on activated
clays is reported by Tsai et al. (
2003
). The rate constant increases with an increase
in salts in the aqueous paraquat solution: from 0.046 (g/mg/min) at a NaCl con-
centration of 0.05 M, to 0.059 (g/mg/min) at a solution concentration of 2.50 M
NaCl. Studying the effect of various alkali metals ions on paraquat adsorption
capacity on activated clay surfaces, Tsai et al. (
2003
) found the order Li
+
(32.79 mg/g) [ Na
+
(31.25 mg/g) [ K
+
(24.75 mg/g). Moreover, the rate con-
stant appears to be inversely proportional to the adsorption capacity.
Competitive adsorption on sepiolite clay of a monovalent dye (e.g., methyl
green or methyl blue) and of the divalent organo-cationic herbicides diquat and
paraquat was studied by Rytwo et al. (
2002
). To evaluate a possible competitive
adsorption between the two organic compounds, separate aqueous solutions of
each cation were used and adsorption isotherms were obtained. Figure
8.28
shows
the amount of diquat, paraquat, and methyl green adsorbed on sepiolite as a
function of total added divalent cation. It may be observed that, when the added
amounts were lower than the CEC of the sepiolite (0.14 mol
c
/kg), all cations were
completely adsorbed.
Rytwo et al. (
2002
) also found that, when the added amounts were higher than
the CEC, the adsorbed amounts of methyl green and diquat increased up to 140