Geoscience Reference
In-Depth Information
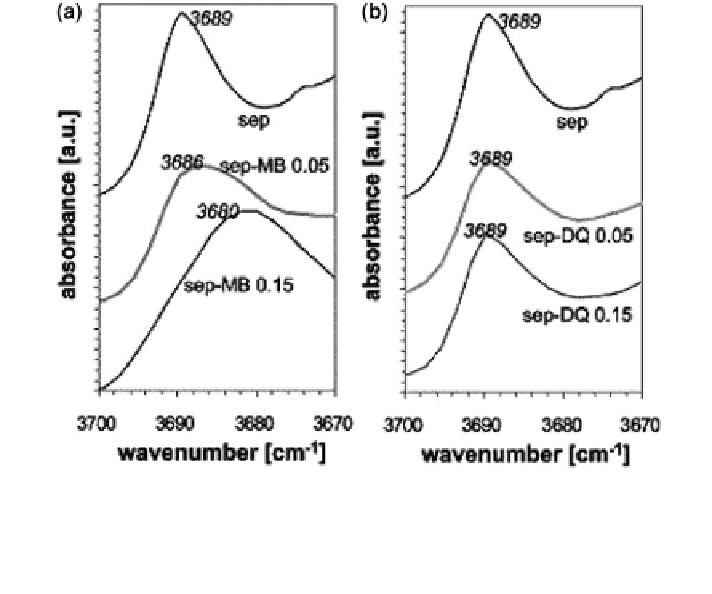
Fig. 8.29 IR spectra of the Si-OH vibration in a pure sepiolite (sep), sepiolite with 0.05 and
0.15 mol
c
methylene blue (MB)/kg clay (sep-MB 0.05 and sep-MB 0.15, respectively) and b pure
sepiolite (sep), sepiolite with 0.05 and 0.15 mol
c
diquat/kg clay (sep-DQ 0.05 and sep-DQ 0.15,
respectively). Reprinted from Rytwo et al. (
2002
). Copyright 2002 with permission of Elsevier
and 115 % of CEC, respectively, while paraquat adsorption reached only a value
close to the CEC level. These behaviors were examined using Fourier transform
infrared (FTIR) measurements. Pure sepiolite exhibits two clear peaks at 789 and
764 cm
-1
. Adsorption of monovalent organic cations such as methylene blue
(MB) leads to a considerable deformation of the peaks at high adsorption loads,
but the peaks are not affected when the adsorption is smaller than one-third of the
CEC. A divalent cation compound such as diquat or paraquat does not affect the
O-H doublet at any adsorbed load. When both diquat and MB are coadsorbed up
to CEC, the peak deformation appears but is ascribed to the binding of the
monovalent organic cation to the neutral sites. Similar effects are observed for the
main Si-OH vibration at 3,700 cm
-1
. The effects of adsorption of MB and diquat
on sepiolite IR spectra are presented in Fig.
8.29
. With increasing amounts of
adsorbed MB, the peak shift is observed (Fig.
8.29
a); no shift is observed when
diquat is adsorbed (Fig.
8.29
b).
Rytwo et al. (
2002
) show that diquat and paraquat adsorb on neutral sites of
sepiolite; the authors speculate that this might be a general pattern for organic
cation contaminants interacting with sepiolite.
Competitive adsorption on clay and soil surfaces between a heavy metal con-
taminant (Cu) and a cationic herbicide (chlordimeform) was reported by Maqueda
et al. (
1998
) and Undabeytia et al. (
2002
). In the presence of herbicides, Cu